The Karl Munger Lab
Oncogenic activities of high-risk alpha genus Human Papillomaviruses (HPVs)
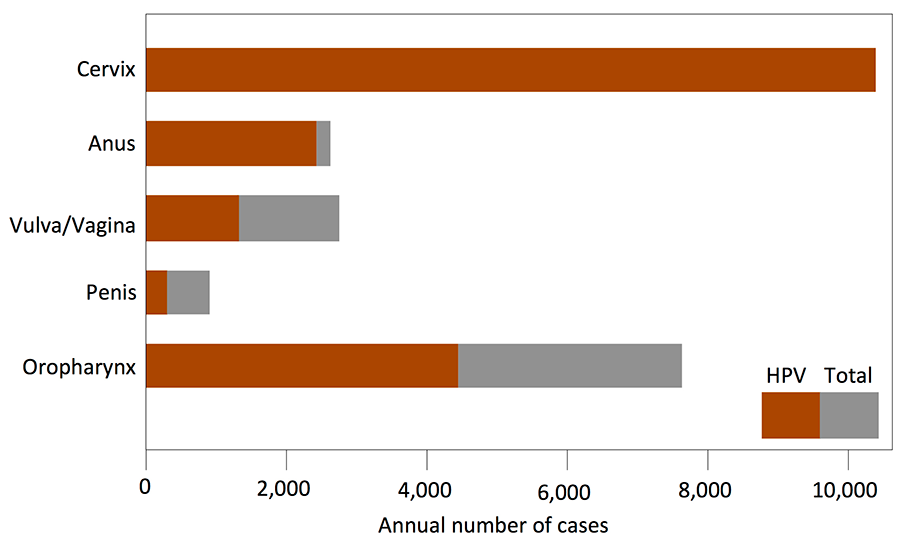
Alpha genus HPVs specifically infect mucosal epithelia and are classified as low-risk or high-risk based on their propensity to cause lesions that can undergo malignant progression. Approximately 5% of all human cancers are caused by high-risk alpha HPV infections. HPV-associated cancers include cervical cancer, a leading cause of cancer death in women worldwide, as well as other anogenital tract and head and neck carcinomas (Fig 1).
Figure 1: Annual Incidence of Major Cancer types associated with HPV infections. (Data from: Cancer 2008; 113: S3036-46)
While cervical cancer rates have decreased due to better preventive screening, the incidence of HPV-associated anal and head and neck carcinoma is on the rise.
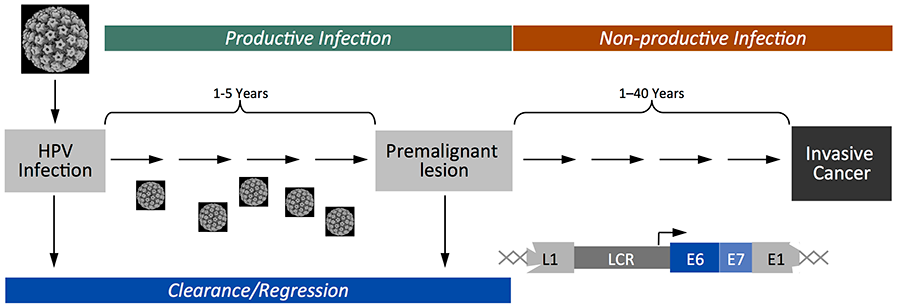
HPV associated cancers generally represent “non-productive” infections; viral proteins are expressed but no infectious virus is produced. Viral genome integration is a hallmark of malignant progression and as a consequence E6 and E7 are the only viral proteins that are expressed in cancers (Fig 2).
Figure 2: Most high-risk alpha genus HPV infections spontaneously regress but persistent infections give rise to premalignant lesions that can progress to invasive carcinoma. HPV associated cancer often only express E6 and E7.
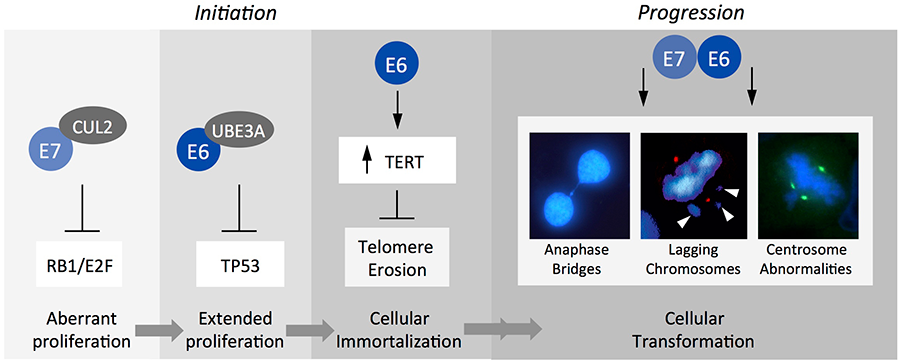
Our research group is determining the transforming activities of the high-risk alpha HPV E6 and E7 oncoproteins at a molecular level with the goal to identify druggable targets. E6 and E7 lack intrinsic enzymatic or specific DNA binding activities. The two proteins are necessary for induction and maintenance of the transformed state and function by subverting cellular signal transduction pathways. While some of these pathways have been delineated at a molecular level, many others remain unknown.
Figure 3: Cellular targets of high-risk alpha HPV E7 and E6 include the retinoblastoma (RB1) and p53 (TP53) tumor suppressors, respectively, which are targeted for proteasomal degradation. E6 also activates telomerase (TERT) activity and targets cellular PDZ domain containing proteins (not shown). Moreover, E6 and E7 contribute to malignant progression by subverting mitotic fidelity thereby triggering genomic instability.
Given that E6 and E7 are small proteins of approximately 150 and 100 amino acids in size, these two viral proteins have evolved to target signaling pathways that modulate multiple downstream targets and effectors (Fig 4).
Figure 4: Molecular classes of HPV E6 and E7 effectors and targets currently studied in the lab.
E6/E7 protein targets
We have been using proteomic and other biochemical analyses to determine previously unknown protein targets of the HPV E6 and E7 oncoproteins, to validate these targets using biochemical and biological assays, and to determine the molecular consequences of such interactions.
E6/E7 non-coding RNA targets
To study HPV E6/E7 modulation of noncoding RNA expression, we have been performing “deep” sequencing of small RNAs and identified several micro RNAs that are specifically dysregulated by E6/E7 expression in undifferentiated human epithelial cells. We are currently investigating mechanisms of induction and downstream targets of these RNAs. Other classes of noncoding RNAs are also under study.
E6/E7 mediated epigenetic alterations
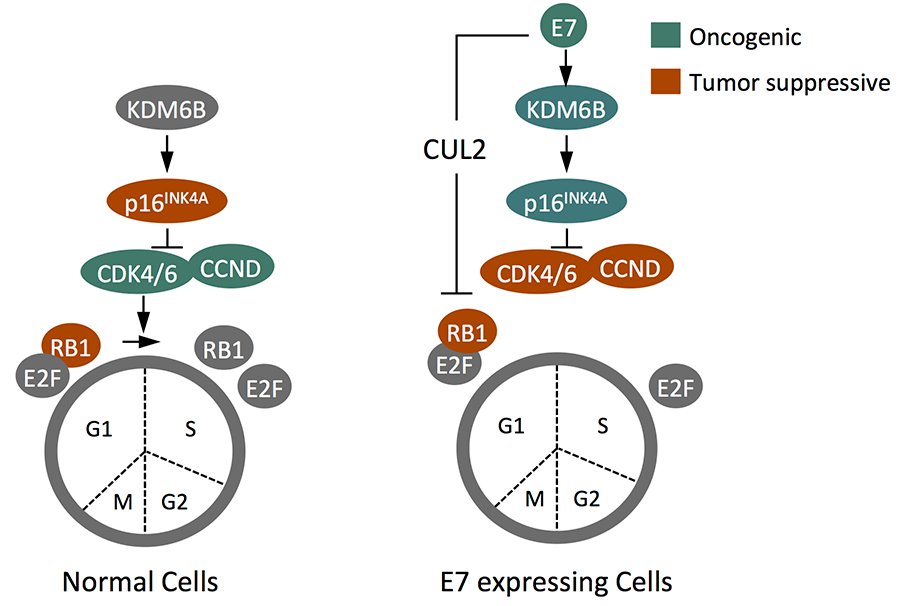
We have found that HPV16 E7 expression causes upregulation of the two histone H3 lysine 27 (H3K27) specific demethylases KDM6A and KDM6B. This results in a global decrease of the repressive H3K27 trimethyl (H3K27me3) mark and epigenetic reprogramming as evidenced by aberrant expression of homeobox genes. We also determined that the p16INK4A cyclin dependent kinase 4 and 6 inhibitor and tumor suppressor is de-repressed as a consequence of KDM6B induction, and that E7 induces this through a pathway that that is similar to a cellular defense pathway that triggers premature senescence in response to cell stress, including oncogenic stress. Since RB1 is a necessary mediator of stress induced cellular senescence, E7 mediated RB1 degradation has evolved to counter this cell defense pathway and HPV16 E7 not only causes aberrant S-phase entry but also alterations in cell identity. Interestingly, KDM6B and, paradoxically, the p16INK4A tumor suppressor become necessary for proliferation/survival of HPV16 E7 expressing cells (Fig 5).
Figure 5: HPV16 E7 triggers p16INK4A through an epigenetic pathway that involves the KDM6B H3K27 demethylase and is to induce premature, RB1 mediated senescence. The ability of E7 to target RB1 for degradation has evolved from the need to overcome this cellular defense response. As a consequence of RB1 degradation, however, p16INK4A and KDM6A expression are required for survival of E7 expressing cells.
Interestingly KDM6A is also essential for survival of HPV E7 expressing cells, although the downstream mediator(s) is/are currently unknown. A KDM6 selective small molecule inhibitor causes cell death in cervical cancer lines, suggesting that these and/or other epigenetic enzymes may be targets for epigenetic therapies. We are currently exploring whether E6/E7 expressing causes alterations in other epigenetic programs.
Biology and Biochemistry of cutaneous HPVs
Most HPVs infect cutaneous epithelia and cause skin warts that are largely benign. In some special populations, such as patients suffering from the rare inherited disorder Epidermodysplasia Verruciformis (EV), or patients under long-term immune suppression, warts caused by infection with some beta genus HPVs can progress to squamous cell carcinomas (SCCs). Since these cancers frequently arise at sun exposed areas of the body, UV exposure has been strongly implicated as a co-factor for HPV associated SCC development. While beta HPV E6 and E7 have potent intrinsic oncogenic activities in transgenic mouse models, their expression is not maintained in every tumor cell and hence, E6/E7 may not be required for the maintenance of the transformed state.
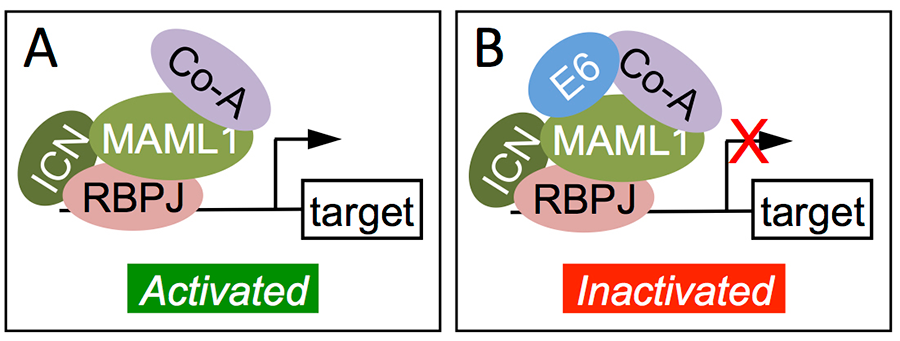
Proteomic experiments have revealed that the beta HPV E6 proteins do not target p53 for degradation but strongly associate with the histone acetyl transferase EP300, some human homologs of the transcriptional co-activator Mastermind (MAML), which function in NOTCH signaling, and the SMAD2/3 transcriptional modulators of transforming growth factor (TGF) β signaling. As a consequence beta HPV E6 proteins inhibit NOTCH and TGFbeta signaling, which are well-established tumor suppressor pathways in epithelial cells.
Figure 6: Inactivation of NOTCH signaling by beta HPV8 E6. E6 associates with and inhibits the DNA bound activating transcription factor complex that contains the intracellular NOTCH fragment (ICN) bound to the DNA binding component RBPJ, the transcriptional coactivator MAML bound to other co-activators (Co-A). [Adapted from J Virol 2013; 87: 4762-7]
We are currently investigating other cutaneous HPVs to determine whether these pathways are regularly targeted by cutaneous HPVs. In collaboration with Dr. Lambert at the University of Wisconsin, we are also investigating a recently isolated cutaneous papillomavirus that infects mice (MmuPV1) to establish an infectious model of HPV pathogenesis in its natural host.