The Giuseppina Tesco Lab
BACE1 and Alzheimer's Disease
Alzheimer’s Disease (AD) is the most common form of dementia that affects 5.3 million Americans. In a small percentage (>1%) of cases AD is inherited as an autosomal dominant trait (Familial AD), however the majority of cases are sporadic. A key neuropathological event in AD is the cerebral accumulation of Aβ, a ~4kDa peptide derived by serial proteolysis of the amyloid precursor protein (APP) by β- and γ-secretase. Beta-site APP-cleaving enzyme (BACE1) is a membrane-tethered member of the aspartyl proteases that has been identified as β-secretase. Several studies have shown that BACE1 protein levels and β-secretase activity are increased in AD brains. Thus, BACE1 elevation may be the first step in increasing Aβ and triggering AD pathology, at least in the sporadic cases.
Our studies have elucidated a novel post-translational mechanism of regulation of BACE1 mediated by the BACE1-interacting molecule, GGA3 (Golgi-localized γ-ear-containing ARF binding protein 3). We have determined that GGA3 depletion stabilizes BACE1 and increases β-secretase activity.
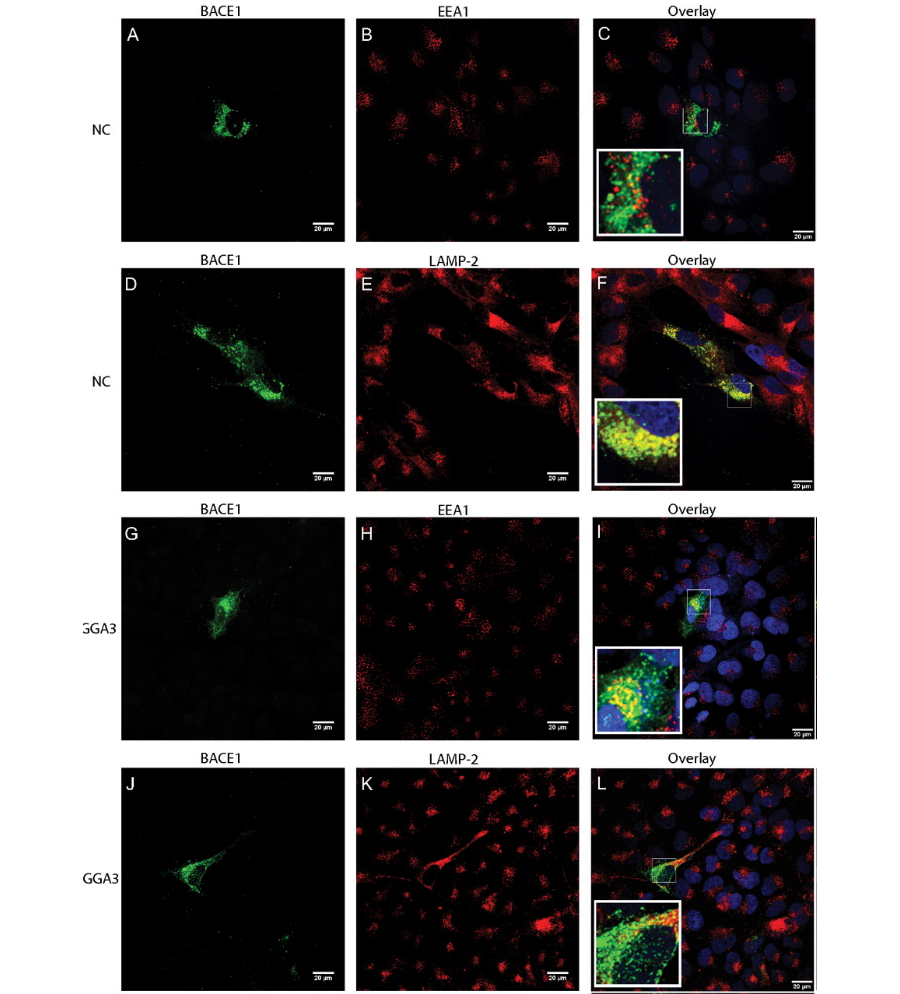
Figure 1. Depletion of GGA3 results in BACE1 accumulation in early endosomes because of impaired delivery of BACE1 to late endosomes/lysosomes.
We also found that levels of GGA3 are decreased in post-mortem AD brains and are inversely correlated with BACE1 levels. We have shown that BACE1 is degraded via the lysosomal pathway and demonstrated that GGA3 regulates the delivery of BACE1 to the lysosomes. The BACE1-C-terminal fragment (CTF) contains a specific di-leucine (DXXLL) sorting signal that has been shown to bind the VHS domain of the three members of the GGA family of proteins, GGA1, 2, and 3. We have found that, unexpectedly, direct binding of GGA3 VHS domain to the BACE1 di-leucine motif is not necessary for this regulation. Instead, GGA3 interaction with ubiquitin is essential for regulating BACE1 levels. Accordingly, we have found that BACE1 is mainly mono- and K63-linked polyubiquitinated at lysine 501. Our work suggests that the impairment of BACE1 degradation is the underlying mechanism of BACE1 elevation in the brains of subjects affected by AD.
BACE1 as a Therapeutic Target
BACE1 is a primary drug target for AD therapy. However, in the decade since the discovery of β-secretase, the identification of effective BACE1 inhibitors that are active in the CNS has been very difficult. The catalytic site of BACE1 is exceptionally long and it has been very challenging to develop small compounds that efficiently inhibit BACE1, are able to cross the blood brain barrier and are reasonably stable.
An alternative approach to BACE1 small-molecule inhibitors is the indirect inhibition of BACE1 through the modulation of regulatory mechanisms that control BACE1 levels or BACE1 trafficking to acidic compartments where it is normally active. Our studies have elucidated a novel post-translational mechanism of regulation of BACE1 mediated by GGA3 and ubiquitin, which represent a potential therapeutic target for the treatment of AD.