The Yongjie Yang Lab
Despite astrocytes have been recognized as the essential component in functional synapses and actively modulate various physiological processes in the brain, how astrocytes acquire their unique morphology and functions during development remains essentially unknown. Potential astrocyte subtypes in the mammalian central nervous system also remain uncharacterized. Lack of this knowledge has become a significant hurdle in understanding astrocyte dysfunction in neurologic disorders/diseases. Astrocytes have been clearly implicated in several neurological diseases/disorders, significantly influencing the progression of these diseases. My lab is currently focusing on the regulation of astroglial glutamate transporter (EAAT2/GLT1) expression; Neuron-dependent regulation of astrocyte maturation during development and in adult brain; Molecular mechanisms of astrocyte dysfunction in pathogenesis of neurodevelopmental disorders (fragile X syndrome) and motor neuron degeneration (amyotrophic lateral sclerosis). Research in my lab will provide novel knowledge about the EAAT2/GLT1 regulation and astrocyte maturation, as well as unveil the mechanisms for the pathological alterations of neuron and astrocyte interaction in neurological disease, Ultimately, it will help develop astroglia-based neuroprotective strategies for these diseases/disorders.
You can learn more about the Yang lab and their work by visiting the Yang lab website and reading below.
Molecular mechanisms of astrocyte maturation and function
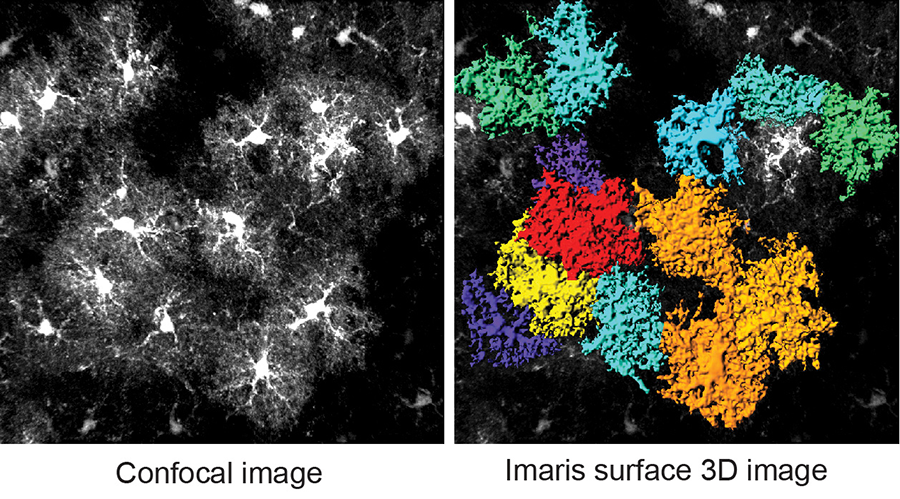
Despite the importance of astrocytes in the CNS, how they become developmentally mature, especially the acquisition of their unique morphology and interaction with synapses/vasculatures and induction of astroglial specific functional genes, has remained essentially unknown. My lab is interested in the molecular mechanisms how astrocytes become functionally mature during postnatal development, especially the role of neuronal signals in astrocyte maturation. To answer these questions, we employ molecular (TRAP and Crispr/Cas techniques), imaging (in vivo astrocyte labeling and morphology analysis), genetic (Cre-loxP/AAV), and electrophysiological (astrocyte patch and dye-filling) approaches, complemented with in vitro primary astrocyte and neuron co-cultures.
Figure 1. Uniquely ramified cortical astrocyte morphology.
Exosomal signaling in neuron to astrocyte communication and in neurodegenerative diseases
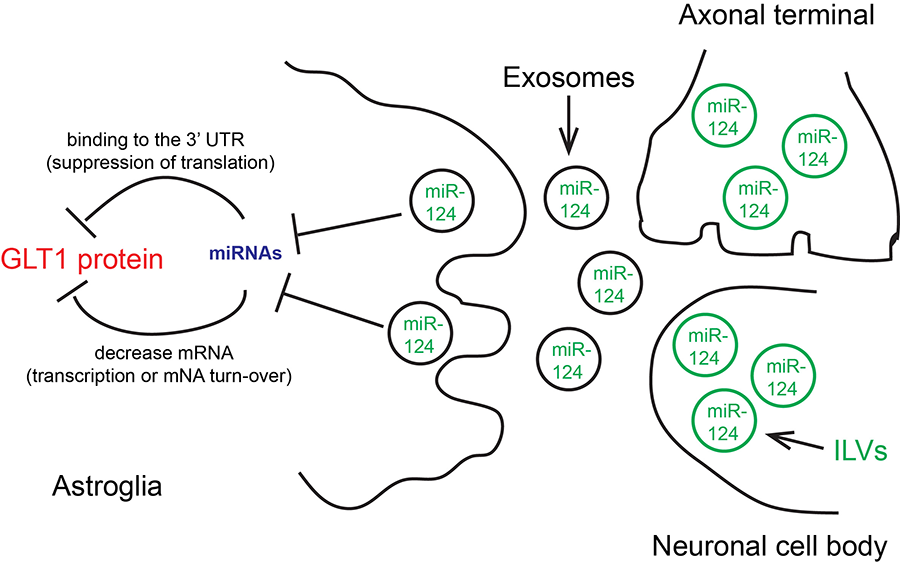
Exosomes, a major type of secreted extracellular vesicles 40-100 nm in size, are derived from intraluminal vesicles (ILV) that are budded inwardly from the early endosomal compartment and are released from multiple vesicular bodies (MVBs), an intermediate endosome structure, during endosome maturation. Recently, exosome-mediated secretion and uptake of disease-causing proteins between CNS cells, including Aβ, α-synuclein, tau, misfolded SOD1 and TDP-43 has been reported. These studies support the hypothesis that exosome-mediated secretion and uptake of disease-relevant proteins could underlie propagation of protein aggregates, which is a widely observed pathological feature in neurodegenerative diseases. We have recently characterized a neuronal exosome-mediated miRNA pathway that regulates translational expression of GLT1 in astrocytes. We have further generated a Cre-dependent exosome reporter mouse line that allows selective labeling of exosomes in specific cell types and tracing of its transfer to receipt cells in vivo. How exosome-signaling is altered in disease and how exosome could mediate disease protein propagation will be investigated using biochemical and imaging approaches.
Figure 2. Neuronal exosomal miRNA-dependent regulation of EAAT2/GLT1 in astrocytes.
Astrocyte dysfunction and pathogenesis of Fragile X Syndrome (FXS)
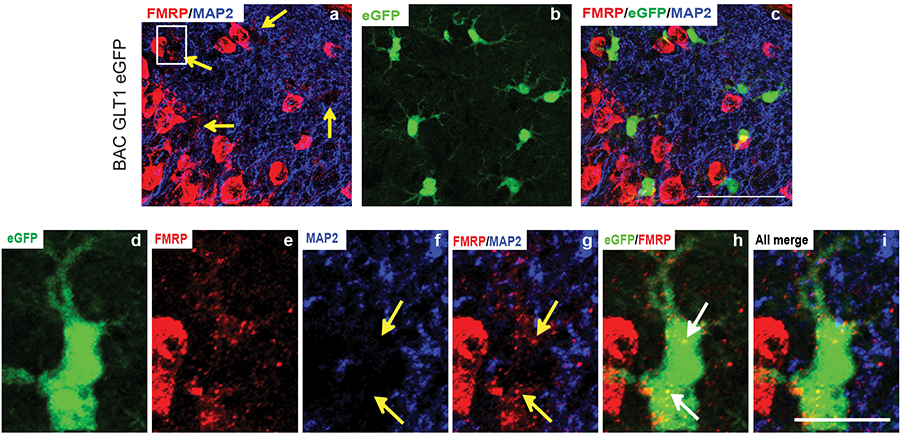
Fragile X syndrome (FXS) is an inherited neurodevelopmental disorder, caused by the loss of function of FMRP. FXS closely resembles autistic syndrome. Functions of FMRP in glial cells remain largely unknown. In addition, little is known about the non-cell autonomous pathogenic mechanisms, -the role of astroglia in the pathogenesis of FXS. We will employ a combination of molecular (TRAP-based profiling), genetic (FMRP conditional knock-out and restoration), biochemical, and electrophysiological approaches to investigate the role of FMRP in astrocytes and how astrocytes contribute to the pathogenesis of FXS. We have recently demonstrated a unique activation role of FMRP in regulating protein expression in astrocytes. We found that astroglial glutamate transporter subtype GLT1 and glutamate uptake is significantly reduced in cortex of the fragile X mice (Fmr1 KO and cKO mice), contributing to enhanced cortical neuronal excitability. We are actively investigating how astroglial FMRP modulates the physiological and molecular changes in astrocytes, which subsequently contributes to disease pathology.
Figure 3. Expression of FMRP in mature cortical astrocytes in vivo.