The David Greenblatt Lab
Mechanisms & Consequences of Drug - Drug Interactions
A focus of our work is on the mechanism and consequences of drug-drug interactions. Of particular interest are those interactions caused by ingestion of nutrients, natural products, and herbal medicines derived from plants. A topic of recent attention in the scientific literature and in the public media has been the interactions of grapefruit juice (GFJ) with prescription medicines. GFJ is unique among food products in that it contains a class of compounds known as furanocoumarins (Fig 1), among which is a specific derivative known as 6’,7’-dihydroxybergamottin (DHB). DHB has the property of forming an irreversible complex with Cytochrome P450-3A (CYP3A) isoforms in human gastrointestinal tract mucosal cells, thereby inactivating the enzyme. This process is termed mechanism-based or time-dependent inhibition. In clinical terms, coadministration of GFJ with several prescription drugs that normally undergo first-pass metabolism (presystemic extraction) by enteric CYP3A can lead to reduced extraction of such drugs, and consequently higher plasma concentrations after oral dosage. This in turn has the potential to enhance clinical effects or even produce toxicity.
Figure 1. Structures of the principal furanocoumarin derivatives found in grapefruit juice. The most important CYP3A inhibitors are 6’,7’-dihydroxybergamottin (6,7-DHB) and the Paradisin dimers (Paradisin A as an example). Bergamottin itself, and the degradation product bergaptol, are relatively inactive as CYP3A inhibitors. (Hanley MJ et al, 2011).
Examples of drugs affected by this interaction are: felodipine, midazolam, triazolam, simvastatin, buspirone, oxycodone, and others (Fig.2). The effect of GFJ on metabolism can last for several days after a single exposure to GFJ. This is attributable to the time necessary for CYP3A regeneration after the irreversible inactivation by GFJ.
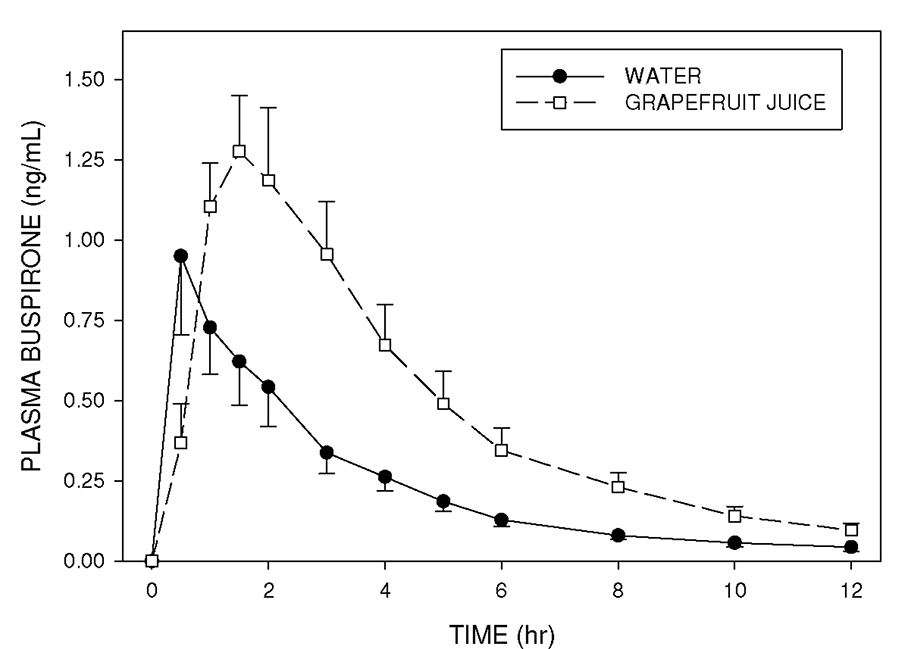
Figure 2. Mean (± SE) plasma concentrations of buspirone following single doses of buspirone administered to healthy volunteers in the control condition (with water), and on a second occasion following ingestion of GFJ. Note the increased plasma buspirone concentrations when the drug is given with GFJ. (Hanley MJ, 2013).
We have an in vitro model of human drug metabolism to study the mechanisms and predictability of drug interactions involving GFJ components and prescription drugs that are metabolized by CYP3A isoforms. The model is based on metabolic activity of the microsomal fractions isolated from human liver samples obtained from potential transplant donors for whom a recipient match was not found. The inhibitory potency of GFJ furanocoumarins is determined from the effect of increasing concentrations of a specific furanocoumarin, such as DHB, on the CYP3A-mediated metabolic transformation of an index CYP3A substrate (Fig. 3).
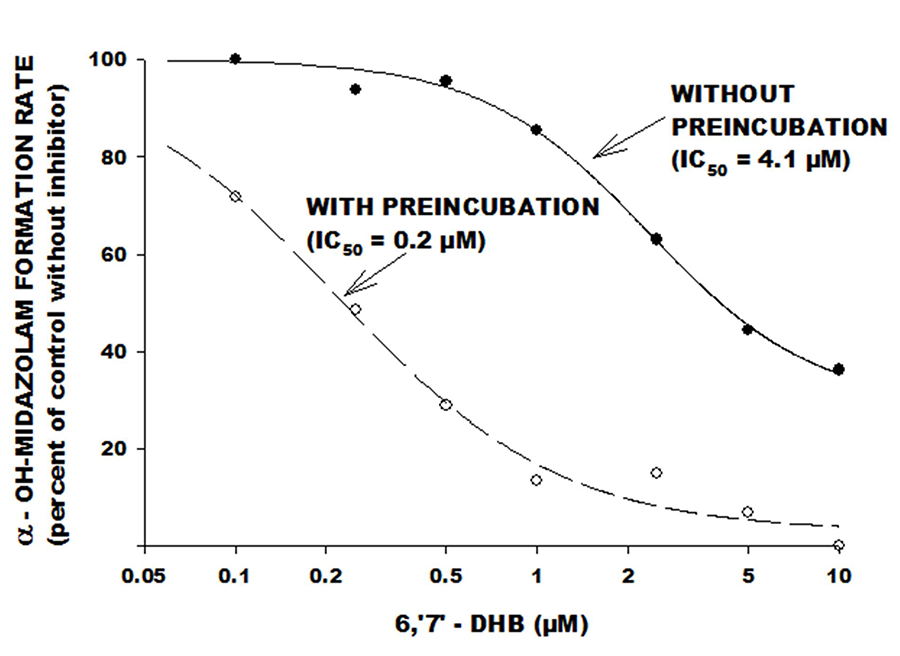
Figure 3. An in vitro study in which 6,7-DHB was tested as a CYP3A inhibitor using midazolam as the index substrate. Increasing concentrations of DHB were incubated at 37°C with a fixed concentration of midazolam, together with drug-metabolizing microsomal protein extracted from human liver. The rate of formation of the principal metabolite of midazolam (Y-axis) decreased as the concentration of DHB increased (X-axis). The 50% inhibitory concentration (IC50) was determined using nonlinear regression analysis. The study was done under two conditions: “without preincubation,” in which substrate and inhibitor were simultaneously added to microsomal protein (closed circles, dashed line); and “with preincubation,” in which the inhibitor was added to microsomal protein prior to addition of substrate (open circles, dashed lines). Note the leftward shift in the curve (lower IC50 indicating increased inhibitory potency) in the “with preincubation” condition – this is consistent with irreversible or mechanism-based inhibition. (Greenblatt DJ et al, 2003).
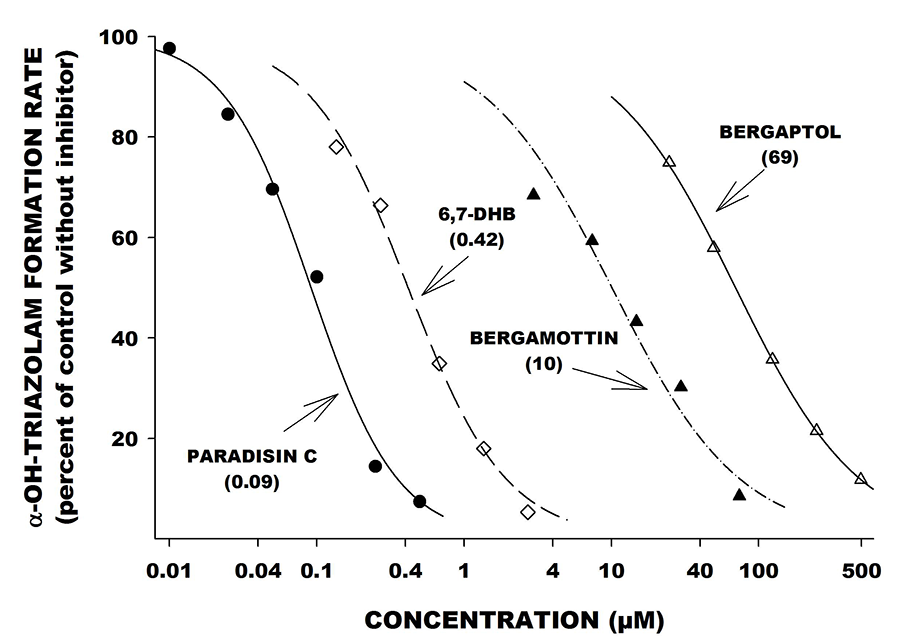
The studies show that DHB is a highly potent CYP3A inhibitor, with the inhibitory mechanism largely explained by an irreversible or mechanism-based process. Even more potent than DHB are a series of dimers (also termed Paradisins) formed by head-to-head or head-to-tail dimerization of DHB with itself, or with bergamottin (Fig.4). Bergamottin itself, and a monohydroxylated breakdown product called bergaptol, are relatively inactive as CYP3A inhibitors.
Figure 4. In vitro inhibition curves for furanocoumarin derivatives found in grapefruit juice: Paradisin C, 6,7-DHB, bergamottin, and bergaptol. All studies were done in the “with preincubation” condition as described in Figure 3. The index substrate in this study was triazolam. Note that Paradisin C and 6,7-DHB are the most potent CYP3A inhibitors (smallest IC50 values). Bergaptol and bergamottin are much weaker inhibitors.
Many GFJ products are commercially available, and not all are equivalent with respect to their capacity to produce drug interactions. Differences can be seen according to the geographic site of the grapefruit-bearing trees, the climate, the season and method of harvest, and the method of storage. In biochemical terms, differences can be traced to variations in content of DHB and the Paradisin dimers. We have worked with agricultural geneticists at the University of Florida who are aiming to develop GFJ hybrids with greatly reduced furanocoumarin content. The objective is to generate GFJ product with a reduced liability to produce drug interactions.