The Alexei Degterev Lab
The Many Faces of Regulated Cell Death: Apoptosis, Necrosis, Necroptosis, Pyroptosis, Ferroptosis and Others
For many years, cell death has been conventionally categorized into either regulated cell death, i.e. genetically encoded and intrinsically regulated process of apoptosis, or spontaneous, stress-induced, unregulated necrosis, which lacks a specific underlying mechanism and represents a catastrophic cell loss under acute pathologic settings. However, this notion has been recently challenged by the discoveries from our and other laboratories, suggesting that necrotic death can result from an array of specific, highly regulated cellular mechanisms. These pathways are mechanistically distinct from apoptosis, but, similarly to apoptosis, may serve critical physiologic roles and contribute to a variety of human diseases.
One such necrotic mechanism was termed "necroptosis". It is induced by a variety of signals (eg, ligands of death receptors (TNFα, FasL, TRAIL), interferons, bacterial and viral pathogen-associated molecular patterns (PAMPs) including Toll-like receptor agonists, etc.), triggering the formation of “necrosome” complexes. These complexes, containing homologous Ser/Thr kinasese RIPK1 and RIPK3, promote rapid execution of highly inflammatory and immunogenic cell death, involving rapid plasma membrane lysis, oxidative stress and release of a variety of pro-inflammatory molecules.
The necrosome pathway appears to play important roles in innate immune responses to bacterial and viral pathogens through the regulation of cell death, inflammatory gene expression, and inflammasome activation. Furthermore, inhibition of necroptosis has shown tremendous promise as a new therapeutic strategy against a variety of human autoimmune and inflammatory diseases (e.g. multiple sclerosis, arthritis, Crohn's disease, psoriasis), neurodegenerative conditions (e.g. Alzheimer's disease and ALS), and acute injuries (e.g. stroke, myocardial infarction and traumatic brain injury). In cancer, activation of necroptosis has been proposed to contribute to the tumor suppression in some cases, and to promote tumorigenesis in others.
Besides necroptosis, the existence of a variety of other non-apoptotic regulated cell death mechanisms is starting to be appreciated, including caspase-1-mediated inflammatory necrotic death termed "pyroptosis", lipid-peroxidation-induced cell death mechanisms termed “ferroptosis” and others. Overall, my lab is interested in both understanding the roles of these mechanisms in normal physiologic innate immune responses, and in finding new ways to either inhibit or induce these responses under pathologic conditions, such as sepsis, multiple sclerosis, intestinal inflammation and brain trauma, to achieve therapeutically beneficial outcomes.
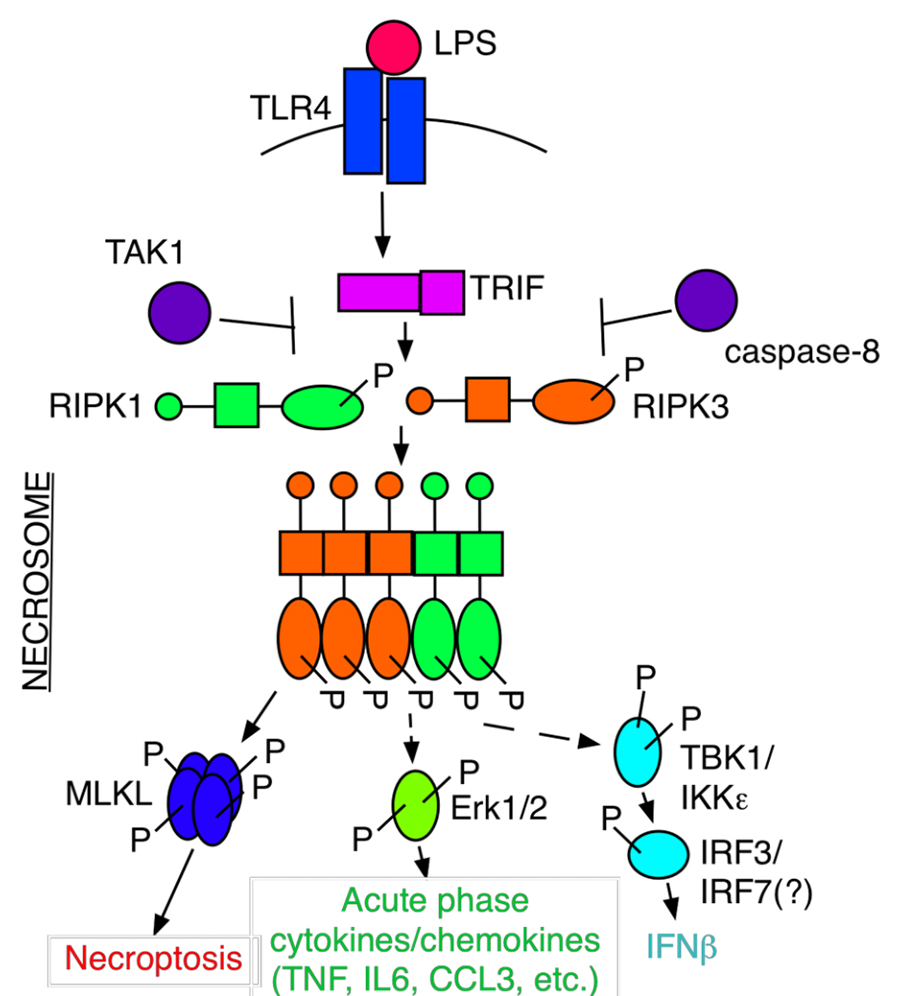
Figure 1. The necrosome is a signaling platform for necroptosis and inflammation.
Necrosomes – Key Mediators of RIPK1 and RIPK3-dependent Responses
Activation of necroptosis involves formation of large detergent-insoluble necrosome aggregates of homologous RIPK1 and RIPK3 kinases. On the one hand, necrosomes provide signaling platforms enabling RIPK3 to phosphorylate a critical necroptosis effector MLKL and execute necroptosis. On the other hand, necrosomes possess a variety of additional, but still poorly understood functions, including activation of a variety of anti-microbial and anti-viral gene expression programs, regulation of metabolism, control of both canonical and non-canonical inflammasome-dependent responses, and, even, induction of apoptosis. All of these mechanisms are differentially activated by necrosome components in a highly signal and cell type-dependent manner. One long-term interest of my lab is to elucidate the composition of necrosome complexes under different circumstances using a variety of proteomics and functional genomics approaches, aimed at understanding both the mechanisms of differential regulation of necrosome-dependent responses, and identification of new targets for small molecule drug discovery.
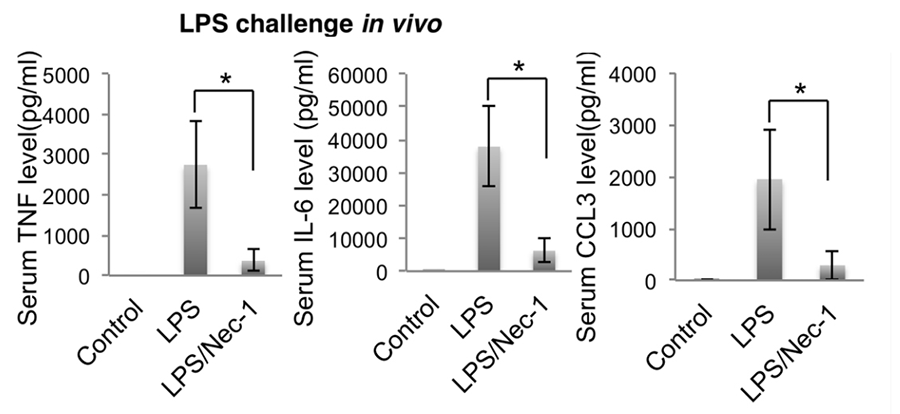
Figure 2. Nec-1s, a small molecule inhibitor of RIPK1 developed by our lab, efficiently inhibits cytokine production in mice challenged with the TLR4 agonist lipopolysaccharide (LPS).
Chemical Inhibitors and Activators of Regulated Cell Death and Associated Inflammation
An important focus of my lab is to develop chemical tools to both interrogate and therapeutically target cell death mechanisms. We design, synthesize, optimize and characterize biological activities of small molecule modulators of key cell death regulators in collaboration with medicinal chemists, X-ray crystallographers and molecular modelers. For example, some of the projects focus on the development of new classes of selective small molecule kinase inhibitors. Currently, we are pursuing development of a number of different chemical series targeting RIPK1, RIPK3 and RIPK2 kinases, aimed at inhibition of PAMP-induced necroptosis and peptidoglycan-driven inflammation, respectively. Additionally, we have identified activators of RIPK1 and RIPK3 kinases, which we are developing to selectively trigger protective pro-death and pro-inflammatory responses in the pathogen-infected cells, cancer cells or tumor-associated stromal cells. In another approach, we are developing molecules targeting key anti-oxidant mechanisms elevated in cancer cells and intrinsically linked to the development of anti-cancer drug resistance with the goal of being able to induce highly selective oxidative death of the chemotherapy-resistant cancer cells.
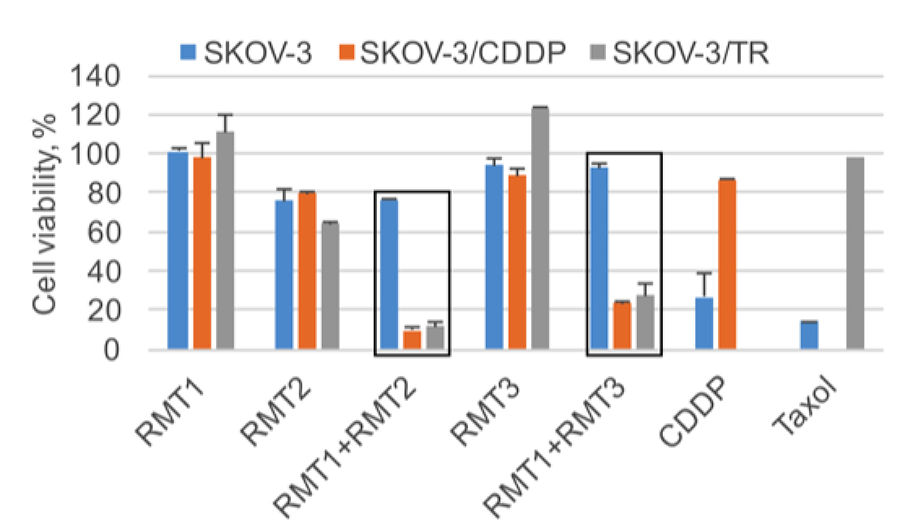
Figure 3. Combinations of Ros-modifying therapeutics (RMTs) induce highly selective oxidative death in ovarian carcinoma cells, resistant to cisplatin (CDDP) and taxol (TR).
Understanding the Roles of RIPKs and Other Associated Factors in Responses to Bacterial and Viral Pathogens
RIPK-containing signaling complexes represent critical regulatory nodes in innate immune responses to bacterial and viral pathogens. In one line of work, we are examining the activation of RIPK1 and RIPK3-dependent responses by a sub-set of gram negative bacteria species, such as K.pneumonaie and P.gingivalis. These projects seek to identify mechanisms employed by the bacteria to suppress protective RIPK-dependent responses. Conversely, we are using a variety of chemical tools, developed by our lab, to activate or restore activation of RIPK responses to promote bacterial clearance. Similarly, we are focusing on the roles of caspase-8-dependent apoptosis and RIPK3-dependent necroptosis in the responses to various DNA viruses, such as Kaposi’s sarcoma-associated herpesvirus (KSHV), and RNA viruses, including HIV and influenza. The goals of these projects are to understand the cross-talk between viral factors, innate immune sensors and caspase-8/RIPK1/RIPK3 complexes, and to elucidate the strategies to enable efficient activation of RIPK or caspase-8-depent host protective responses during infection.