The Stephen Moss Lab
Neuronal Communication and GABA Receptors
Neurons communicate with each other via synaptic transmission, a process that is fundamental for information processing in the brain and that underlies complex neuronal phenomena such as learning and memory. Inhibitory neurotransmission is largely mediated by gamma-aminobutyric acid (GABA) and is critical for sculpting complex patterns of neuronal activity. Deficits in inhibition result in numerous neuropsychiatric disorders that include autism, anxiety, addiction, cognitive deficits, depression, epilepsy, and schizophrenia.
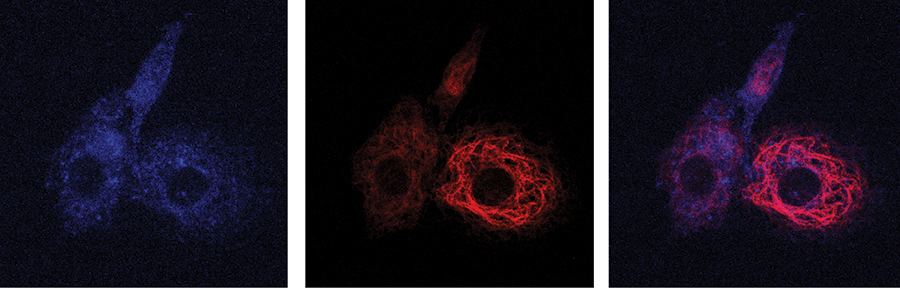
Figure 1.Cell surface (blue) and internalized (red) GABAA alpha4 subunit in COS-7 cells. Overlay shows no colocalization.
The fast inhibitory actions of GABA are mediated by GABAA receptors, which are Cl--selective ligand-gated ion channels. Their activation in the adult brain leads to Cl- influx, which leads to reduced levels of excitation. GABAA receptors are also the sites of action for benzodiazepines, barbiturates, neurosteroids and general anesthetics, all of which act to potentiate receptor activity and affect the efficacy of inhibitory synaptic transmission.
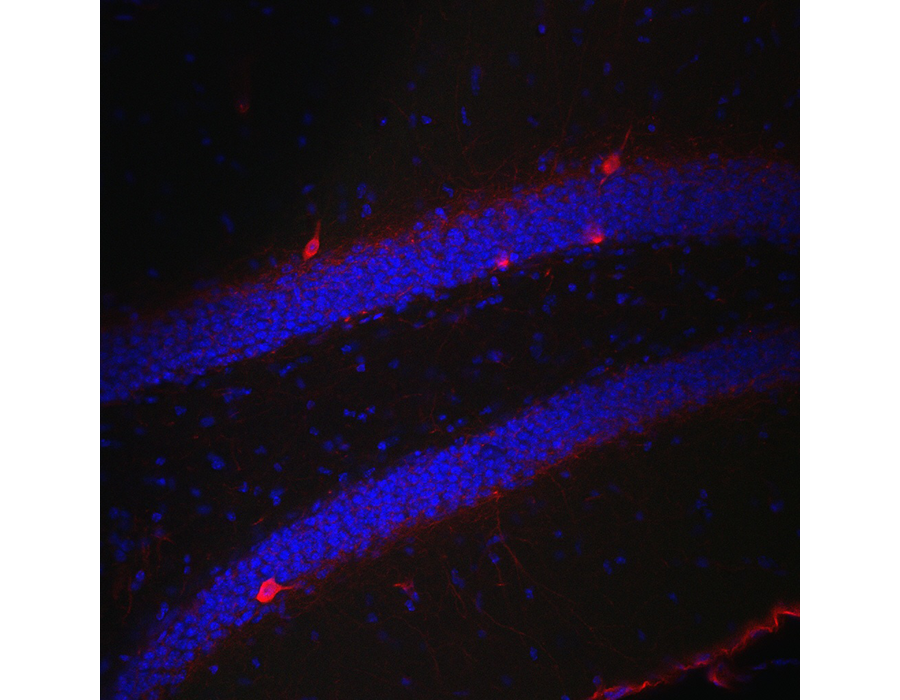
Figure 2. Parvalbumin (red) and DAPI (blue) staining in the dentate gyrus of an adult mouse.
We are principally interested on understanding the endogenous mechanisms by which neurons control the activity of GABAA receptors and how these processes determine the level of neuronal activity together with behavior. Our studies have revealed that GABAA receptor activity is subject to dynamic modulation.
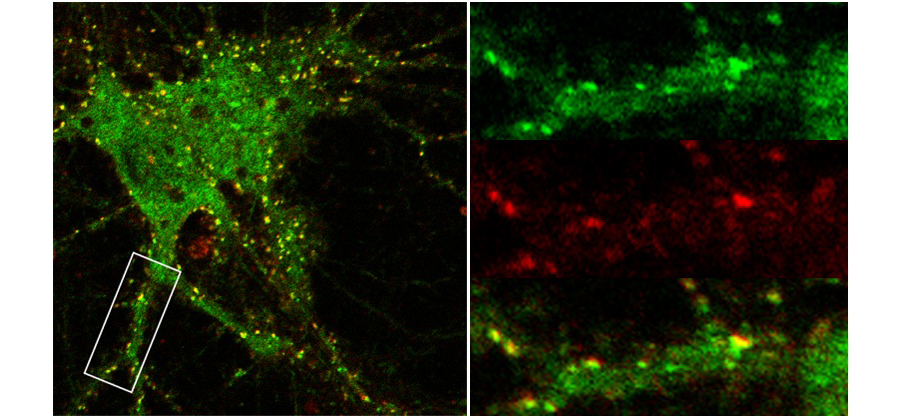
Figure 3. Dynamic trafficking of GABAA receptors in rodent hippocampal neurons. Green = total GFP-tagged GABAA receptors (includes cytoplasmic and surface receptor populations); Red = surface GABAA receptors inserted in 10 minutes @ 37˚C
Covalent modification of GABA receptor structure, termed phosphorylation is involved. This phosphorylation can alter both the activity and subcellular localization of these proteins within neurons. To test the significance of these regulatory processes we use genetically engineered mice in which GABAA receptor phosphorylation has been ablated. These mice show deficits in synaptic transmission and alter behavior. Our results suggest that manipulating GABAA receptor phosphorylation may be a useful therapeutic strategy to modify the efficacy of neuronal inhibition and thus may aid in treating a broad range of neuropsychiatric disorders.
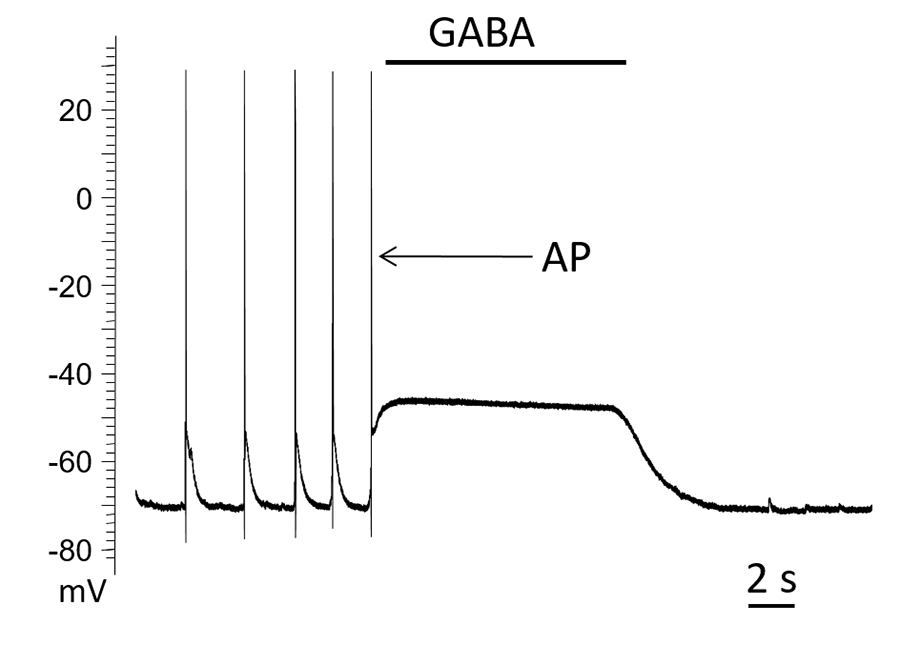
Figure 4. GABA elicits action potentials (AP) in hippocampal neurons after treatment with glutamate. Prior to glutamate application, GABAergic transmission was hyperpolarizing.