The Brent Cochran Lab
Brain Tumor Stem Cells
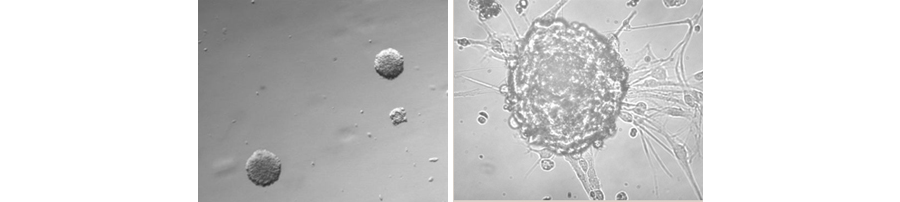
Glioblastoma multiforme (GBM), grade IV, is the most common type of brain cancer with the worst prognosis. The average post-operative survival after tumor resection is less than 14 months. Due to the highly lethal nature of glioblastoma, new therapies are urgently needed. Recent work suggests that tumor stem cells drive some tumor and represent a new therapeutic target for cancer. Some of the best evidence for this hypothesis comes from studies of glioblastoma. Only a few percent of glioblastoma cells have stem-like properties, but essentially all of the tumor-initiating ability resides with these cells. These cells are highly efficient at forming tumors requiring only a few hundred cells for tumor initiation, whereas traditional glioma cell lines typically require 100,000 cells or more to initiate a tumor in mice.
Figure 1. Formation of neurospheres from primary GBM cells.
Interestingly, the tumor stem cells have properties similar to neural stem cells. They form neurospheres in serum-free media and can be induced to differentiate into neurons, astrocytes, and oligodendrocytes. On the other hand, they differ from normal stem cells in that they can be passaged indefinitely in culture and carry some hallmark oncogenic mutations such as p53 and Rb. Histologically and by gene expression analysis, the human GBM stem cells produce tumors in mouse brains that are much more similar to human GBM than are serum derived glioma cell lines derived from the same tumors. Stem cell-derived tumors are more invasive and heterogeneous than tumors derived from glioma cell lines. Glioblastomas that harbor neurosphere forming stem cells have a worse prognosis than tumors that don't harbor these cells. Thus, at minimum GBM stem cell lines are better models of human glioblastoma than are traditional serum grown cell lines. The hope and promise of these findings are that novel therapies could be developed that specifically target tumor cells and would, therefore, be more effective than therapies that target the bulk of the tumor cells. Early experiments along these lines confirm this idea.
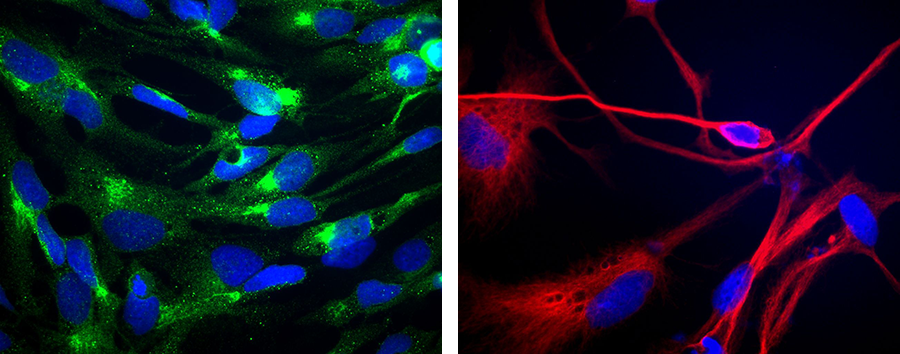
Figure 2. (Left) Staining of GBM stem cells with anti-CD133 (green) – a stem cell marker. Nuclei are stained blue. (Right) Staining of differentiated GBM stem cells with the neural marker beta-III-tubulin (red).
My lab is studying the molecular basis of how “stemness” is maintained in GBM stem cells. The transcription factor family now known as STATs for “signal transducers and activators of transcription” was initially described in my lab. This transcription factor family shuttles between the cytoplasm and nucleus in response to tyrosine phosphorylation to regulate gene transcription. The STAT3 protein is activated in many cancers including glioblastoma. We have shown that STAT3 function is required for the maintenance of stemness and proliferation in glioblastoma stem cells. We are currently studying the mechanisms by which STAT3 does this and are now investigating the regulation of the stem cell epigenome by STAT3. We have also conducted RNAi and small molecule screens of GBM stem cells in order to identify new therapeutic targets and are investigating how these stem cells regulate tumor angiogenesis. We are also using bioinformatics approaches to model GBM signaling networks in silico.