The Amy Yee - K Eric Paulson Lab

HBP1, Wnt Signaling and Breast Cancer
The core of our laboratory is devoted to understanding how HBP1 and Wnt signaling regulate the proliferation and invasiveness that characterizes most breast cancers. We have implemented and developed relevant cell and animal models to address the molecular questions. We use clinical specimens to complement the molecular and cellular studies. To address the important problem of recurrence, we have used statistical and bioinformatics tools to interrogate databases and develop new hypotheses for investigation. Our goal is to develop mechanism-based pre-clinical models that recapitulate aspects of human breast cancer. These models should be valuable for evaluating new compounds and new therapeutic strategies with established compounds in clinical trials. This multidisciplinary approach provides an excellent opportunity to delineate the complex processes for invasive breast cancer and provide new insights into new therapeutic strategies for definitive breast cancer treatment.
The HBP1 Transcriptional Repressor and Breast Cancer
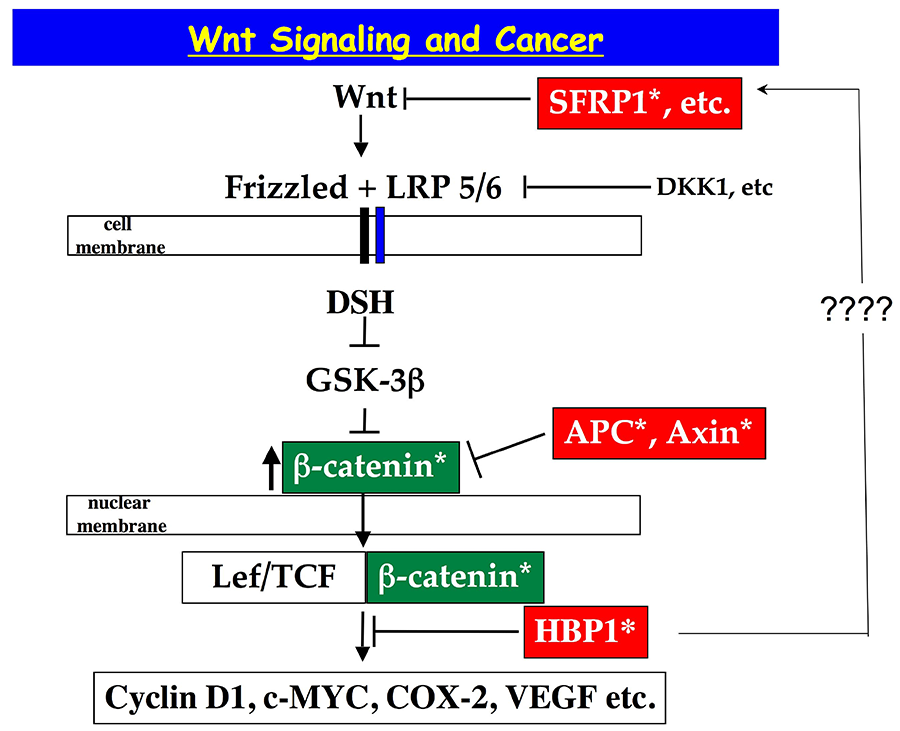
HBP1 is a transcriptional repressor of the HMG box family, of which other HMG box transcriptional activators are linked to Wnt signaling (LEF,TCF). The previous work by our lab and others highlight a role for HBP1 in the suppression of Wnt signaling and of cell proliferation.
Figure 1. The Wnt signaling pathway illustrating the key role played by HBP1 in regulating downstream events in the Wnt pathway.
Clinical Correlations to Invasive Breast Cancer
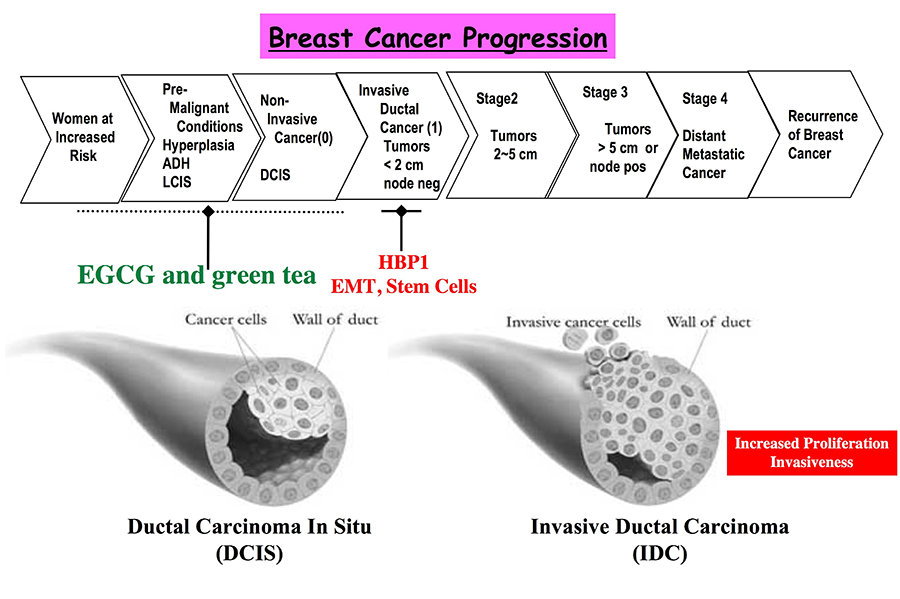
Our work indicates a direct clinical link from aberrations in HBP1 specifically to invasive breast cancer, of which increased proliferation and invasiveness are salient features. The completed studies indicate that HBP1 and its pathways may have a critical role for invasive breast cancer onset and in determining recurrence. We published that HBP1 mutations and reduction are associated with invasive breast cancer. Subsequent functional studies revealed that reduction of HBP1 levels increased breast tumorigenic proliferation and invasion in both cell and animal models. To address relapse, we showed that reduced HBP1 expression and co-reduction of HBP1 and of SFRP1 in the primary breast tumors predicted an exceptionally poor relapse free-survival. Thus, the HBP1 mechanisms with regards to SFRP1 may play a central role in defining the acquisition of invasiveness and ultimately in determining prognosis for relapse-free survival.
Figure 2. The drawing depicts steps in breast cancer progression and highlights stages that are addressed in our experiments.
Self-Renewal, Epithelial-to-Mesenchymal Transitions (EMT), and Invasiveness
A new project seeks to understand the relationship of stem cells to the acquisition of invasiveness. Recent work has shown that the EMT and self-renewal processes are linked together. Work in other labs now indicate that Wnt signaling may specify renewal of stem cells that are prone to invasiveness. We are currently investigating the role of HBP1 in specifying and EMT-stem cell-like transition. We also seek to expand the resident signaling network to identify potential drug targets for invasive breast cancer with respect to HBP1 and this unusual transition. These studies potentially may uncover new drug targets to block mammary tumor cell renewal and to prevent recurrence.
Because of a role for invasive breast cancer, we are using the 3D mammary cell models to investigate the role of HBP1 in setting up an invasive morphology. Our initial efforts are directed at the consequences of HBP1 decreases and/or a hyperactive Wnt signaling pathway in determining an invasive morphology. These studies will also provide a different way to investigate EMT transitions that are thought to precede invasive breast cancer. We are using the MCF10A-erbB2-based 3D mammary models and which shows an excellent cooperation with Wnt signaling in specifying an invasive morphology. Previous work by Brugge and co-workers have indicated that this 3D model mimics the pathology of a high grade DCIS that is prone to relapse to invasive disease. Increased Wnt signaling decreased SFRP1 or decreased HBP1 all specify an invasive morphology, but only in collaboration with activated Her2 signaling. These results predict that heightened Wnt signaling or the decreased HBP1/SFRP1 may specify relapse to invasive disease.
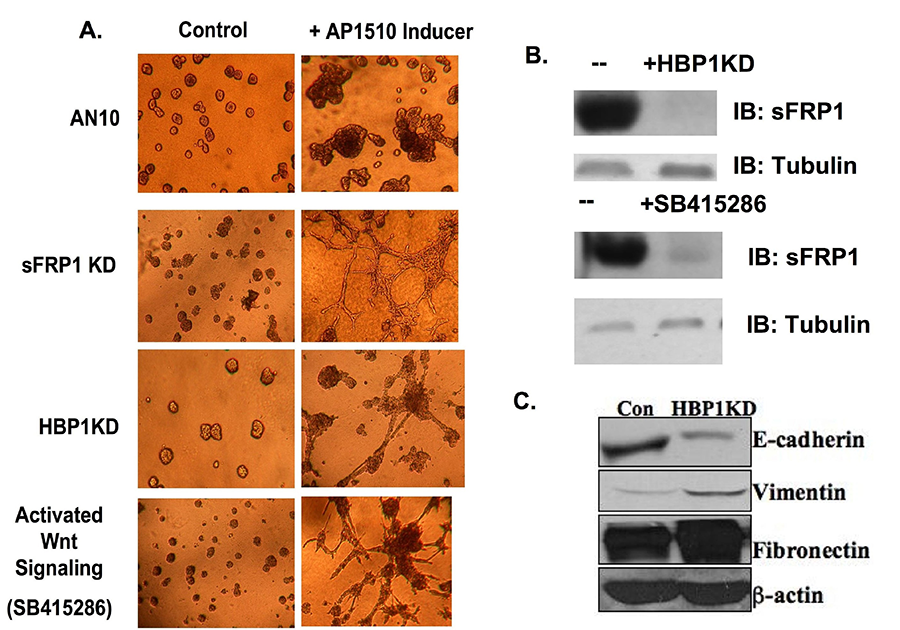
Figure 3. Wnt and HER2 signaling and Invasion in 3D models. (A) The indicated AN10 cell derivatives were placed in 3D matrigel with 1% collagen and then treated with either vehicle or AP1510 to induce Her2 crosslinking for 4 days. All three conditions induce Wnt Signaling. All structures were photographed at day 8. (B) The western blot reveals that HBP1-KD or Wnt signaling activation (GSK3β inhibition) decreases SFRP1 expression. (C) HBP1 knockdown in AN10 cells exhibits decreased epithelial marker (E-cadherin) expression and increased mesenchymal marker (vimentin, fibronectin) expression, consistent with an EMT-like phenotype.
Epigenetic Regulation, Wnt Signaling and HBP1
Our recent studies have begun to elaborate an epigenetic mechanism to regulate Wnt signaling. The SFRP1 gene is an inhibitor of Wnt signaling and a target of epigenetic regulation through histone methylation (H3K27me3). There is a striking association between SFRP1 decreases and invasive breast cancer. Thus, the mechanisms that coordinate epigenetic regulation of SFRP1 are likely to be central to invasive breast cancer. Our preliminary studies show that HBP1 may coordinate both epigenetic events and provide a new mechanism for regulating Wnt signaling. Both H3K27 and DNA methylation (through the EZH2 and DNMT1 genes, respectively) are under investigation. Current efforts are directed at dissecting the HBP1-mediated gene network that coordinates epigenetic regulation of SFRP1 and of Wnt signaling.
EGCG and Green Tea as Tools to Improve Epigenetics-based Chemotherapy
Epigenetics-based chemotherapies may be particularly useful for the spectrum of cancers and recurrences due to constitutive Wnt signaling. We wish to increase SFRP1, DKK1 and other Wnt pathway inhibitors to block Wnt signaling. The strategy is to improve Decitabine, which has known toxicities, by devising mechanism-based combinations that increase efficacy in blocking Wnt signaling and potentially in treating invasive breast cancer. We showed previously that the green tea compound EGCG (Epigallocatechin 3-gallate) blocks Wnt signaling by inducing the HBP1 protein. Thus, we are testing if EGCG can augment epigenetics-based chemotherapeutic drugs such as Decitabine in blocking Wnt signaling in cell-based and in animal xenograft pre-clinical models. The early results indicate that the combination of EGCG and Decitabine demonstrably elevate HBP1 and SFRP1 and suppress breast tumors. EGCG has no known toxicity and is in Phase 1 clinical trials for other cancers. While decitabine is also in Phase 1 trials for solid tumors, its toxicity is well known from the treatment of certain leukemias and myelodysplastic syndrome. Thus, mechanism-based ways to improve efficacy of decitabine may expedite judicious applications for solid tumors.
Wnt Signaling and Epilepsy
Epilepsy is a devastating disease characterized by recurrent seizures, affecting over 3 million Americans, including soldiers who have sustained battlefield traumatic brain injuries. While the precipitating events are usually brain injuries, the initial seizure is termed Status Epilepticus (SE), defined as prolonged and continuous seizure activity, itself can result in significant morbidity and mortality. SE marks the beginning of epileptogenesis, a process leading to spontaneous and recurrent seizures. Remarkably, given the large number of afflicted Americans, epilepsy is classified as a disease of unmet need with a limited repertoire of agents, none of which are anti-epileptogenic in humans. An ideal therapeutic agent could attenuate SE at the time of the initiating event and thus modify the progression to epilepsy. A gap in knowledge in the epilepsy field is that the molecular circuitry underlying the critical periods between initial injury, status epilepticus, and chronic seizure onset is not sufficiently delineated. This lack of knowledge is a barrier to discovering new therapeutic strategies.
We are applying principles of cancer therapeutics/mechanisms to the treatment of epilepsy. The work is a collaborative project between the Yee labs in Boston (Amy Yee, PhD) and in Denver (Audrey Yee, MD). Audrey Yee MD is a clinical neurologist at the University of Colorado Medical School. Using animal models of temporal lobe epilepsy, we have recently discovered that the Wnt signaling network is altered in SE, thus opening new opportunities to translate cancer-related therapies into studies of epilepsy. Wnt signaling is already reported to play a role in normal neuronal development, in neurogenesis and in synaptic plasticity—processes of relevance to epileptogenesis. We reason that attenuation of SE will prevent the still-undefined events in the latent period that results in the onset of chronic seizures. Using our respective expertises in cancer biology and in neurology, we are devising and testing specific cancer drug combinations for intervention in the epileptogenic processes, based on mechanistic considerations.