The William Bachovchin Lab
New Approaches to Cancer and Diabetes Therapies
In the past decade, the focus of cancer therapies has been on "personalized" medicine in which mutated proteins which drive tumor growth are identified through genotyping, and then targeted by various blocking agents. Most often this produces a temporary response followed by relapse, with little overall benefit. Cancer is more genetically diverse, more unstable, and more resilient than previously thought.
Small Molecule Immune Stimulators
We have identified small molecules that can stimulate the immune system to attack tumors. Our lead compound in this class can enhance the efficacy of a dendritic cell cancer vaccine to produce regression and rejection of bulky, established tumors in a preclinical model. As human tumors progress, they develop resistance to immunity. Normally, by the time of a cancer diagnosis, immune resistance has developed, rendering tumor vaccines ineffective. The promising preclinical results suggest that our compounds appear to disarm the shield protecting the tumor from the host immune system. As shown by collaborative studies with the Dr. Terry Fry of the NCI, our agents appear to be especially promising when used in combination with anti-tumor vaccines.
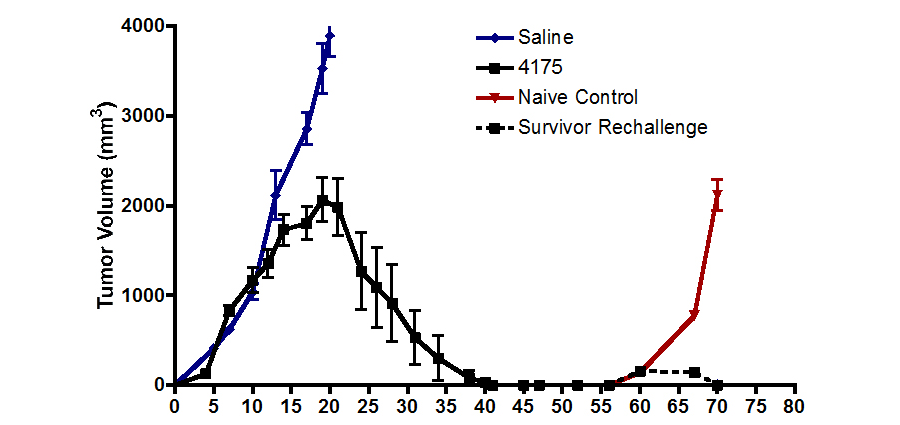
Figure 1. Mouse tumor volume vs days after treatment with ARI-4175 from days 3 thru 39. Rechallenge at day 56 has no further effect, showing complete immunity.
Tumor-activated Small Drugs
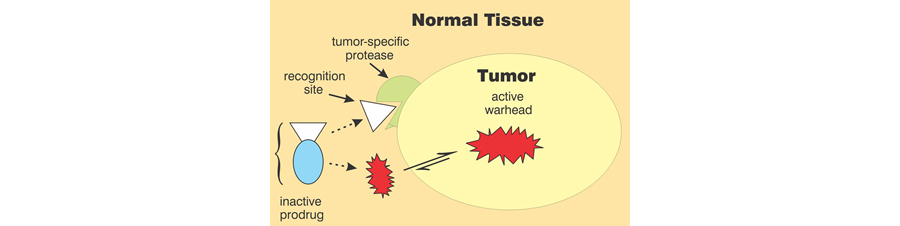
Our prodrug platform also enables the selective delivery of a variety of cytotoxic agents to cancer cells and their supporting stroma, thereby sparing normal cells and tissues from collateral damage. Our current leading prodrug is activated by fibroblast activating protein (FAP) to release a proteasome inhibitor similar to Velcade®.
Figure 2. Strategy for prodrug development.
FAP is a serine protease selectively expressed on the surface of stromal cells surrounding most tumors of epithelial cell origin; but it is not present in most healthy tissues. Activation of the prodrug by FAP directs the proteasome inhibitor to selectively kill the tumor and its supporting stroma. The resulting depletion of the stroma may also lead to activation of the immune system to kill the tumor by relieving the immunosuppressive effects of stromal cells. The released proteasome inhibitor has been designed to inactivate with time so as to further limit exposure of normal cells and tissues to its cytotoxic effects. The lead compound has been demonstrated safe and effective in animal models of pancreatic cancer and multiple myeloma.
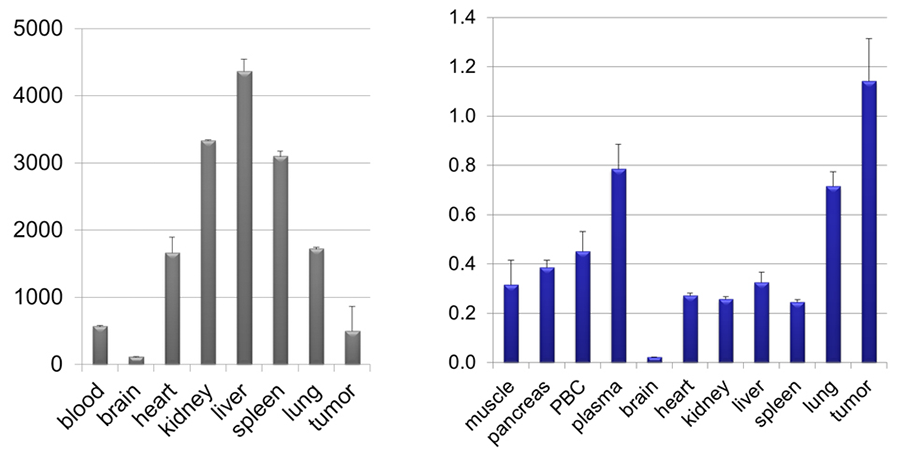
Figure 3. Our "warhead" concentrates in the mouse tumor (right) compared to tissue distribution of Velcade® (left).
Diabetes
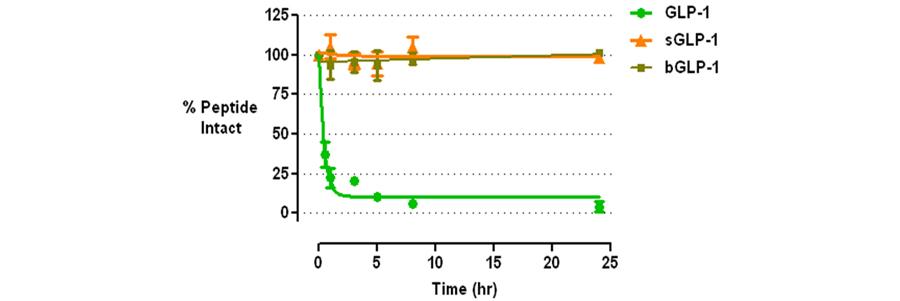
Glucagon-like peptide (GLP-1) is an incretin peptide synthesized by the “L” cells of the intestine in response to food intake. Its biological activities, exerted through the GLP-1 receptor (GLP-1R), include glucose-dependent insulin secretion, slowing of gastric emptying, and inhibition of food intake, stimulation of islet cell proliferation and neogenesis, and restoration of beta-cell sensitivity to secretagogues. The pharmacological potential of these activities for treatment of diabetes has attracted substantial interest. However, GLP-1 is rapidly degraded by dipeptidyl peptidase IV (DPPIV) in vivo, rendering administration of native GLP-1 of limited value. Thus, over the past decade, interest has been directed towards DPPIV-resistant analogs of GLP-1 and inhibitors of DPPIV. Both directions have led to FDA approved drugs. Stable GLP-1 analogs on the market include Byetta® and liraglutide. DPPIV inhibitors on the market include sitagliptin, vildagliptin, and saxagliptin, with several more compounds in late-stage clinical development.
Stable GLP-1 analogs have several advantages over DPPIV inhibitors. These include more effective control of blood glucose, and weight loss. DPPIV inhibitors have the advantage in being orally available, as all previous, stable GLP-1 analogs must be injected. We have developed a platform technology for rendering biologically active peptides such as GLP-1 resistant to any serine protease via minor chemical modifications that leave the character and biological activities of the parent peptide intact. Our lead candidate is a stable GLP-1 analog that is structurally more homologous to native human GLP-1 than Byetta®, but is equally effective in vivo in controlling blood glucose levels. In addition, we have made minor modifications of our lead candidate that renders it orally available. Our research to date indicates that it should combine the effectiveness of the injectable GLP-1 analogs with the ease of use of the oral DPPIV inhibitors.
Figure 4. P1' Modified GLP-1 Analogs Resistant to DPPIV