The Rajendra Kumar-Singh Lab
Understanding Vision and Treating Vision Loss
The focus of our laboratory research is to develop an understanding of the processes of vision and to apply that knowledge towards the treatment of vision loss or blindness. In particular, our laboratory is developing therapies for age-related macular degeneration (AMD), retinitis pigmentosa, uveitis and diabetic retinopathy. Collectively, these are among the most common causes of blindness in the young and the elderly in industrialized nations. Recently, we and others have established the role of an aberrant complement system in the underlying etiology of AMD, diabetic retinopathy and uveitis. Specifically, we have found that the membrane attack complex (MAC) of the complement system is significantly elevated in models of these diseases as well as in patients. Elevation of MAC in ocular tissues leads to the activation of cell signaling and tissue remodeling associated with neovascularization and cell death. We have developed a gene therapy approach using an adeno-associated virus (AAV) to deliver a recombinant inhibitor of MAC known as sCD59 or protectin (AAVCAGsCD59) for the inhibition of MAC in ocular tissues. This approach has been utilized by us to demonstrate proof of principle for the treatment of several ocular diseases including dry and wet AMD as well as uveitis and diabetic retinopathy. We are also currently active in understanding the role of the activated inflammasome and its relationship with the MAC in the above diseases.
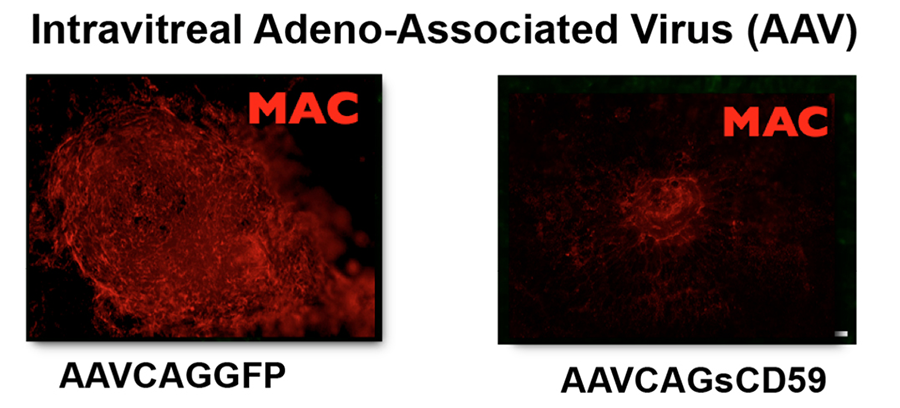
Figure 1. The most commonly utilized model of the wet form of AMD is laser induced choroidal neovascularization. In this model, MAC is deposited at the site of the laser induced damage. In contrast to AAVCAGGFP (a negative control), delivery of AAVCAGsCD59 to the vitreous leads to a significant reduction in laser induced deposition of MAC, here stained red using an antibody against MAC.
These studies have advanced from the bench (in vitro) through proof of concept preclinical studies, IND-enabling toxicology studies, and as of December 2017, a phase I clinical trial examining 17 patients with advanced dry AMD has completed enrolment (ClinicalTrials.gov Identifier: NCT03144999). Pending results, a Phase II clinical trial is planned for late 2018. These latter clinical studies are being funded and performed by Hemera Biosciences Inc. of which the PI is the scientific founder. Our goal in the laboratory and the clinic is to expand use of this and similar approaches to additional ocular diseases.
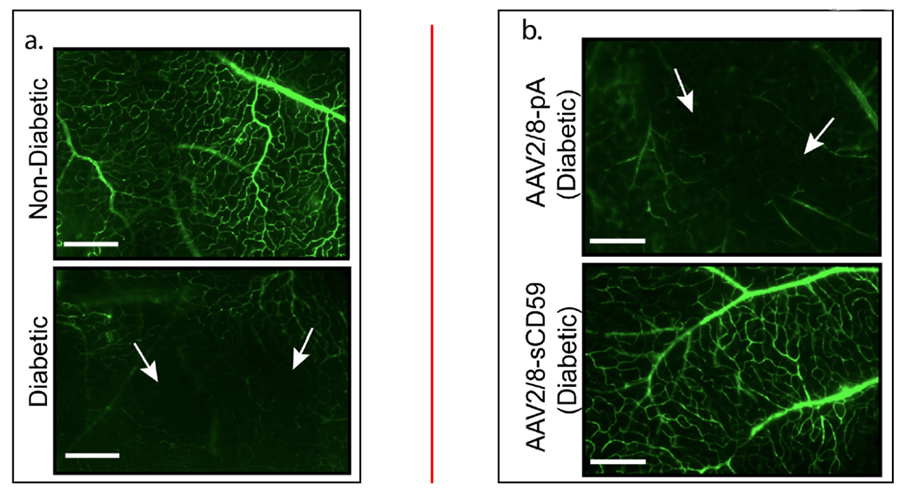
Figure 2. In an acute model of streptozotocin- induced diabetes, we have found that in contrast to negative controls, delivery of AAV2/8-sCD59 (a variant of AAVCAGsCD59) can inhibit non-perfusion of retinal vessels in diabetic retinopathy. Retinal morphology is disrupted in diabetic retinopathy, and AAV2/8-sCD59 can reduce the level of this disruption. Studies are currently under way to measure whether the same therapeutic efficacy can be achieved in chronic models of diabetes.
We have developed several anti-complement molecules that can inhibit activation of complement and deposition of the MAC systemically. These molecules, known as STAC and DTAC can inhibit deposition of human MAC on murine liver, heart and other tissues more effectively than sCD59 because they are comprised of multiple complement inhibitory domains that target various parts of the complement system. These studies are still preclinical, and it is our hope to advance these molecules for the treatment of neurodegenerative diseases. The role of complement in Alzheimer’s, Parkinson’s and other relatively common neurodegenerative diseases is being recently better realized and molecules such as STAC and DTAC may have therapeutic efficacy in such diseases.
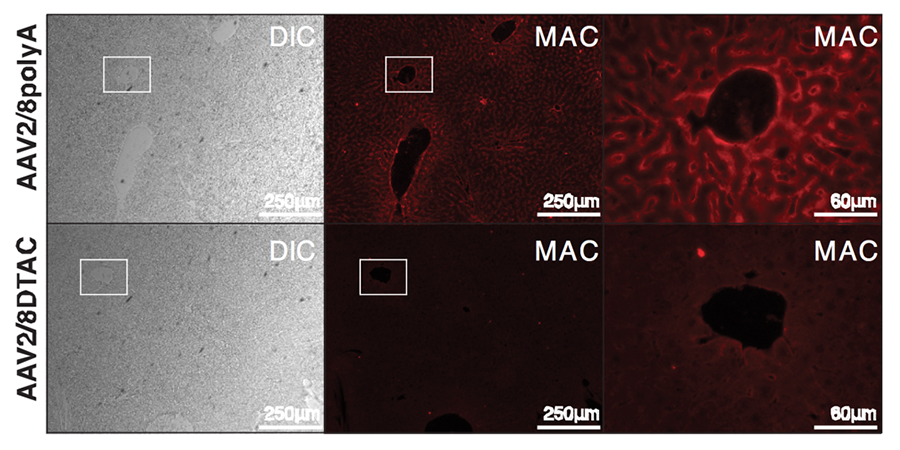
Figure 3. Deposition of human MAC (stained in red using an anti-MAC antibody) on murine liver can be significantly reduced using AAV2/8 vector expressing a recombinant DTAC molecule. From left to right: bright field, MAC and a higher power view of the boxed regions.
We have a very active program for the development of therapies for retinitis pigmentosa (RP)- also a disease with no FDA-approved therapy. We are developing gene editing strategies such as use of CRISPR/Cas9 for replacement of rhodopsin-associated RP. In order to deliver genes and proteins to retinal cells, we develop viral and non-viral vectors specifically for the retina. One currently active project is to develop a platform technology for the delivery of proteins into retinal cells following blast trauma such as to inhibit the process of apoptosis. Blast trauma in the combat theater leads to apoptosis mediated retinal damage. Currently, there is no method available to inhibit apoptosis induced blast trauma in Members of the Armed Forces while in the combat theater. Our laboratory is funded by the Department of Defense (US Army) to develop such a technology. Apoptosis is central also in RP and other retinal diseases and thus our technologies will have application in multiple indications.
In summary, our research is multidisciplinary, ranging from a deeper understanding of the molecular mechanisms of retinal degeneration through proof of concept studies for the treatment of various forms of blindness and application in humans through clinical trials. Graduate students and postdoctoral fellows interested in participating in such research are encouraged to write directly to the PI to check availability of positions in the laboratory that varies based on grant funding. The PIs primary appointment is in the Department of Developmental, Molecular and Chemical Biology with secondary appointments in the Departments of Neuroscience and Ophthalmology. Significant opportunities exist for student exposure to physician scientists and thus MD/PhD students are highly encouraged to apply.
Our research is funded by the National Institutes of Health, The Department of Defense (US Army), several private foundations and generous donors.