The Fair Vassoler Lab
Transgenerational Effects of Opioid Exposure
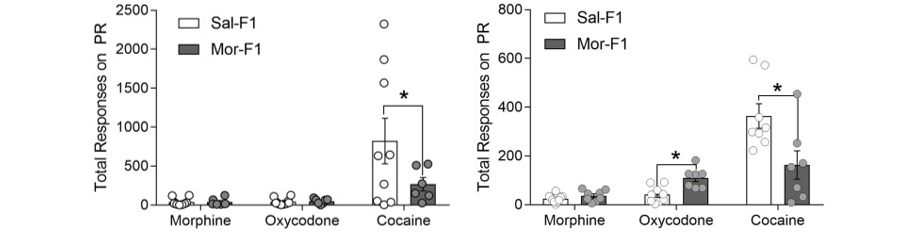
As the United States is in the midst of an opioid epidemic, the levels of exposure to opioids across the population are extremely high. In order to determine the potential impacts that opioid exposure in one generation may have on subsequent generations, the lab utilizes a well-developed model of adolescent morphine exposure. Male rats are administered increasing doses of morphine or saline for a discrete 10-day (PND30-39) window during adolescence. They are then maintained drug free for at least 4 weeks until adulthood. Offspring (F1 animals) are tested for behavioral changes. Recently, we discovered that both male and female Mor-F1 offspring showed increased responding for opioids (morphine and oxycodone) yet decreased responding for cocaine compare to the Sal-F1 control animals. This bidirectional response indicates specific changes in reward circuitry as a result of their father’s adolescent opioid exposure. The progressive ratio (PR) responding for specific drugs is shown in Figure 1.
Figure 1. Progressive Ratio Responding Following Morphine Self-Administration. The responses of male animals are shown on the left and those of female animals are shown on the right. See text for additional details.
Mechanism of Transmission
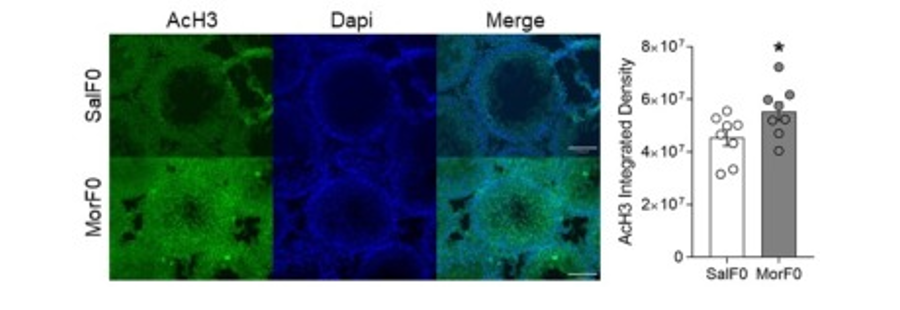
A key goal of the lab is to determine epigenetic mechanisms by which experiences or exposures in one generation can be transmitted to future offspring. We have identified changes in seminiferous tubules and are actively examining spermatozoa as well as blastocysts. The following figure shows acetylated histone H3 protein within seminiferous tubules of F0 males (sires). During spermatogenesis, histones are generally replaced with protamines within spermatozoa, so the retention of acetylated histones may play a role in altering the course of development in the offspring.
Figure 2. Acetylated Histone H3 Expression in Seminiferous Tubules of F0 Males. Changes in histone H3 acetylation are evident in the sires.
Non-pharmacological Therapeutic Modalities to Treat Substance Use Disorder
In addition to understanding the impact that opioids may have on future generations, it is also critical to develop novel treatments in order to help individuals with substance use disorder remain abstinent. We utilize the preclinical model of intravenous self-administration and reinstatement using operant chambers. Previously, we demonstrated that deep brain stimulation (DBS) may be an effective therapeutic modality for the treatment of refractory substance use disorder. In an industry-academic collaboration, we are testing the capacity of a less-invasive strategy to decrease reinstatement of oxycodone seeking. Magnetic nanoparticles (MNPs) situated in deep brain nuclei, are stimulated with an external magnet to produce magnetic mechanical stimulation of adjacent neurons. Preliminary evidence suggests that this may be effective at decreasing levels of drug-seeking behavior.