The John Leong Lab
Interaction of bacterial pathogens with host cells
Our general interest is in the interaction of bacterial pathogens with mammalian cells, interactions that promote colonization and trigger immune responses that can enhance tissue damage and/or result in clearance of the pathogen.
Enterohemorrhagic Escherichia coli
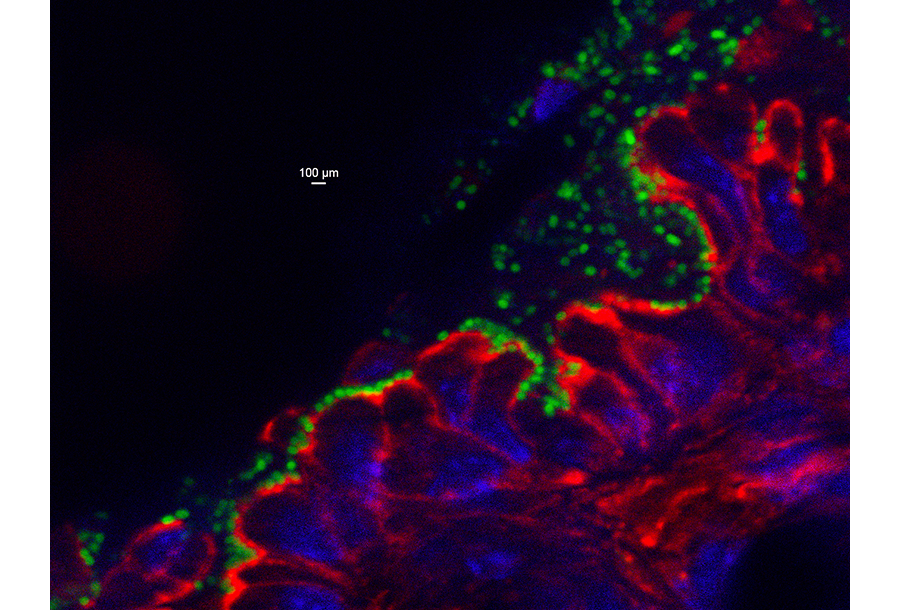
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 utilizes a specialized type III secretion system that injects bacterial “effectors” into mammalian cells to colonize the colonic mucosa, and produces the potent phage-encoded Shiga toxin, which damages the intestinal epithelium and, in some patients, results in systemic vascular damage and renal failure. Antibiotic treatment can induce production of the phage-encoded toxin and is contraindicated during EHEC infection, so we lack specific therapies to prevent or treat EHEC infection and disease. To investigate mechanisms of disease and generate new methods to fight EHEC disease, we utilized the natural murine pathogen Citrobacter rodentium, which colonizes the colonic epithelium similarly to EHEC, to develop a mouse model for EHEC (Fig 1). Our goal is to generate means to block colonization or disease by targeting bacterial or host factors related to the function of the type III secretion system or the production or action of Shiga toxin. These studies may provide approaches to address other intestinal infections or disorders.
Figure 1. C. rodentium colonizes the murine colonic mucosa. GFP-producing bacteria are localized on the epithelium, for which the nuclei are stained blue with DAPI and filamentous actin is stained red with phalloidin.
Borrelia burgdorferi, the Lyme disease spirochete
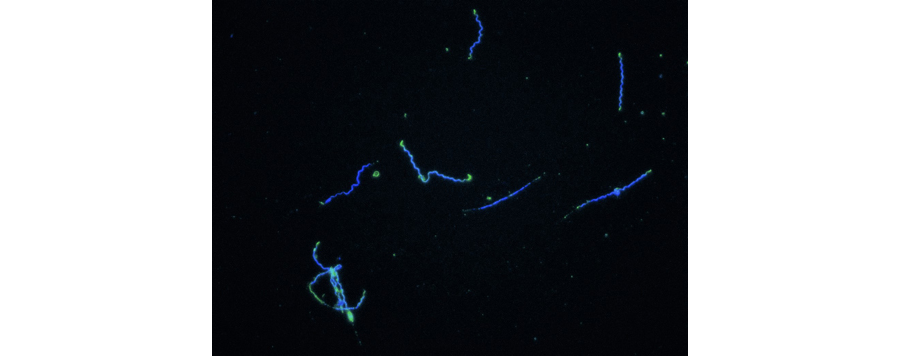
The Lyme disease spirochete Borrelia burgdorferi sensu lato, carried by the Ixodes tick, is the most common vector-borne pathogen in the U.S. In the days to weeks following the establishment of a local skin infection at the site of the tick bite, this bacterium can spread to the heart, joint and other tissues. Lyme disease strains differentially colonize different tissues, leading to diverse manifestations of disease. We utilize a variety of methods, including genome-wide screens and targeted mutagenesis, to identify and investigate spirochetal factors that promote bloodstream survival and tissue colonization with a particular focus on factors that influence dissemination kinetics and the tissue tropism. We have found that the spirochete encodes surface proteins of variable sequence that modulate activation of the host complement system and influence the sites of colonization.
Figure 2. B. burgdorferi produces CRASPs, i.e. surface proteins that bind host complement regulatory proteins. Wildtype B. burgdorferi, stained with Hoechst (blue), bound to FITC-labeled Factor H (green), which typically localizes to the bacterial poles.
Streptococcus pneumoniae
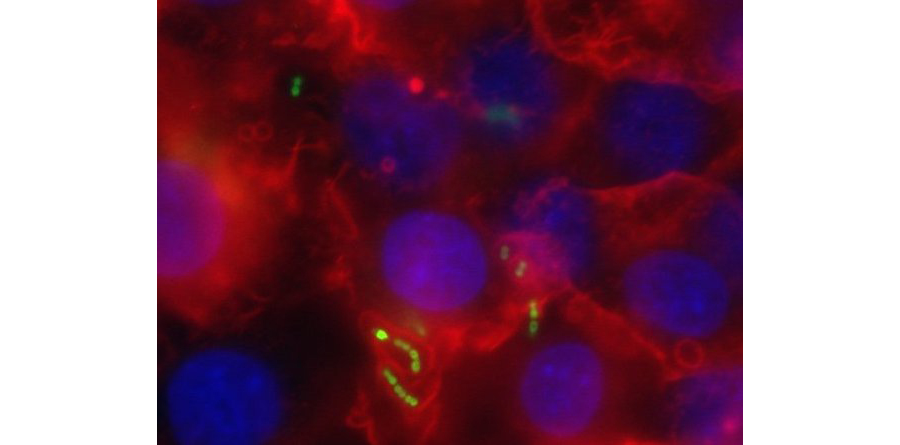
Invasive pneumococcal disease caused by Streptococcus pneumoniae is responsible for over a million deaths per year worldwide. Lung infection, one of the most common forms of invasive pneumococcal disease, is associated with a robust influx of polymorphonuclear cells (PMNs) into alveolar spaces. This acute inflammatory response causes significant tissue damage and contributes to pneumococcal disease. By establishing pulmonary epithelium infection models (Fig 3), we have identified pathways by which S. pneumoniae triggers PMN influx into the airways (Fig 4) and have shown pneumolysin, a cholesterol-dependent pore-forming toxin, induces PMN migration across pulmonary epithelium.
Figure 3. S. pneumoniae (green) infects pulmonary epithelial monolayers. GFP-producing bacteria are localized on the epithelium, for which the nuclei are stained blue with DAPI and filamentous actin is stained red with phalloidin.
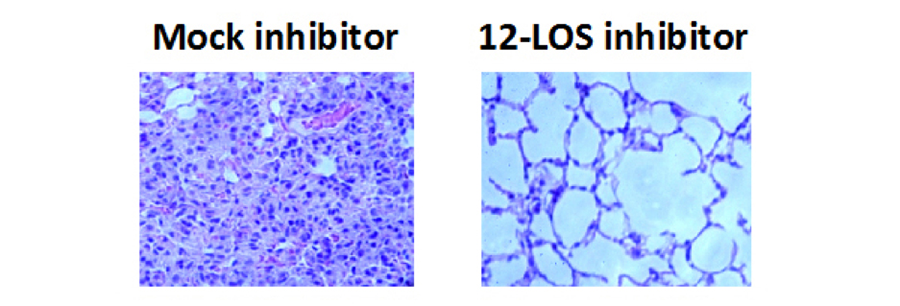
Importantly, neutrophil transmigration severely damages epithelial integrity and promotes the spread of the pathogen into the bloodstream. Thus, mice diminished in the recruitment of PMNs to the airway space are paradoxically protected lethal systemic disease by S. pneumoniae. Finally, this pathogen causes particularly severe disease in elderly individuals and in those co-infected with influenza virus. Inflammatory responses are altered upon aging or viral co-infection, and we are utilizing murine models to investigate these susceptibilities with the goal of mitigating systemic disease.
Figure 4. Mice that had been mock-treated or treated with a 12-LOS inhibitor were infected intratracheally with Streptococcus pneumoniae, and lungs were H&E stained after 2 days of infection.