The Li Zeng Lab
Arthritis and Cartilage Regeneration
Arthritis is a widespread debilitating disease in which joint cartilage is degraded. Due to the limited regenerative ability of cartilage, the cartilage tissue is often permanently lost. Understanding the mechanisms of cartilage development and degradation is essential for devising strategies to slow down arthritis and regenerate cartilage. We study novel regulators of cartilage development and maintenance, as well as develop technologies to facilitate these investigations.
Cartilage Development and Regeneration
Most of the bones in the vertebrate form using the endochondral ossification mechanism, in which a cartilage template is first formed before it is eventually replaced by the bone tissue. Thus it is important to investigate cartilage development to understand bone formation. Cartilage development involves the determination of cartilage cell fate and subsequent chondrocyte differentiation in which proliferating chondrocytes become hypertrophic, forming a distinctive growth plate that drives longitudinal growth.
Cartilage cell fate determination
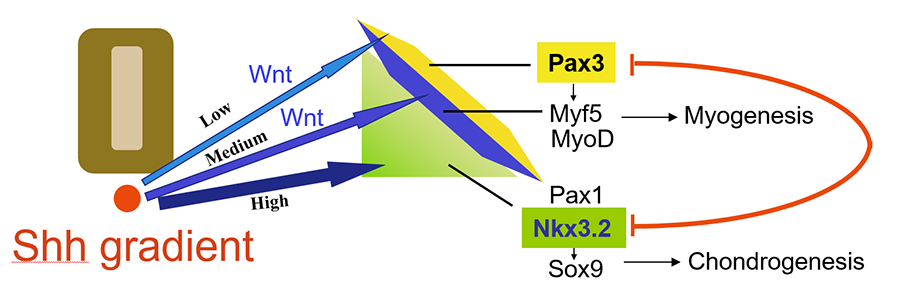
We found that a Sonic hedgehog (Shh) concentration gradient elicits patterns of different cell fates within the somites in a morphogen-like manner, with higher levels of Shh promoting the cartilage cell fate, and lower levels of Shh promoting the muscle cell fate. Additionally, interactions of factors such as Nkx3.2 and Pax3 that are induced by Shh and Wnt signaling further demarcate the fields of muscle and cartilage cells (Cairns et al, 2008; Fig. 1).
Figure 1. Shh and Wnt signaling specify cartilage and muscle cell fate determination in the somite (Cairns et al, 2008).
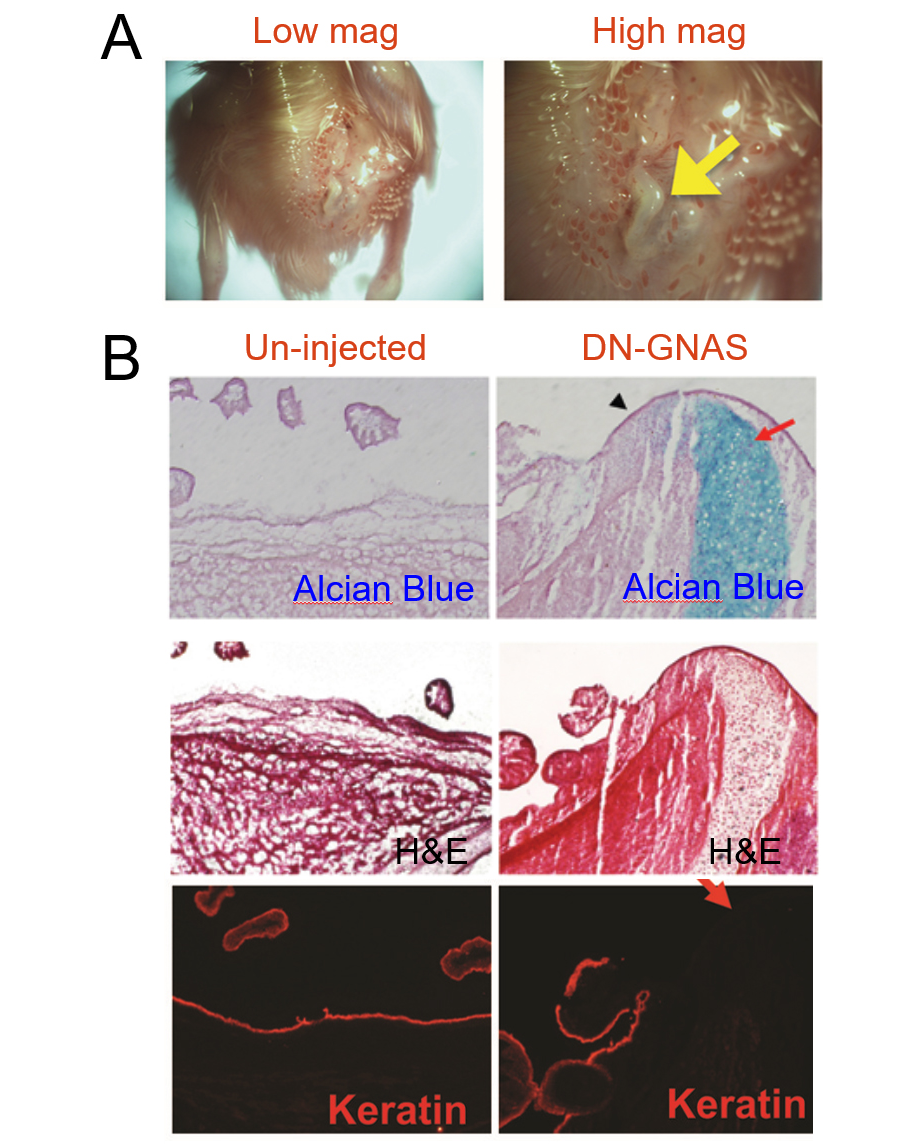
These signaling events are important as their disruption may cause diseases with abnormal skeletal structures. In a collaborative study with Drs. Fred Kaplan, Eileen Shore and Robert Pignolo from University of Pennsylvania, we introduced the dominant negative form of a G protein subunit (GNAS) and observed massive ectopic cartilage and bone formation, mimicking the disease condition of heterotopic ossification (Cairns et al, 2013; Fig. 2).
Figure 2. Ectopic expression of dominant-negative GNAS leads to ectopic cartilage formation (Cairns et al, 2013).
Long Bone Development
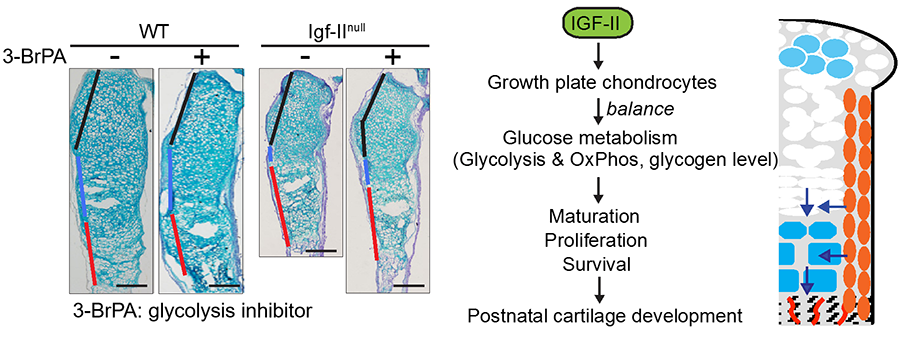
Our study showed that the Insulin-like growth factor II (IGF2) was a critical factor for cartilage and bone development, as Igf2 null mice exhibit abnormal timing of chondrocyte hypertrophy as well as reduced perichondrial growth and differentiation. As a result, the Igf2 null mice have shorter bones, resembling growth arrest in humans due to IGF2 mutations. Interestingly, our biochemical and two-photon imaging analyses identified elevated and imbalanced glucose metabolism in the Igf2 null mouse. Attenuating glycolysis rescued the mutant phenotype of premature cartilage maturation, thereby indicating that IGF2 controls bone growth by regulating glucose metabolism in chondrocytes (Uchimura et al, 2017).
Figure 3. IGF2 promotes cartilage development through regulating glucose metabolism (Uchimura et al, 2017
Arthritis and Inflammation
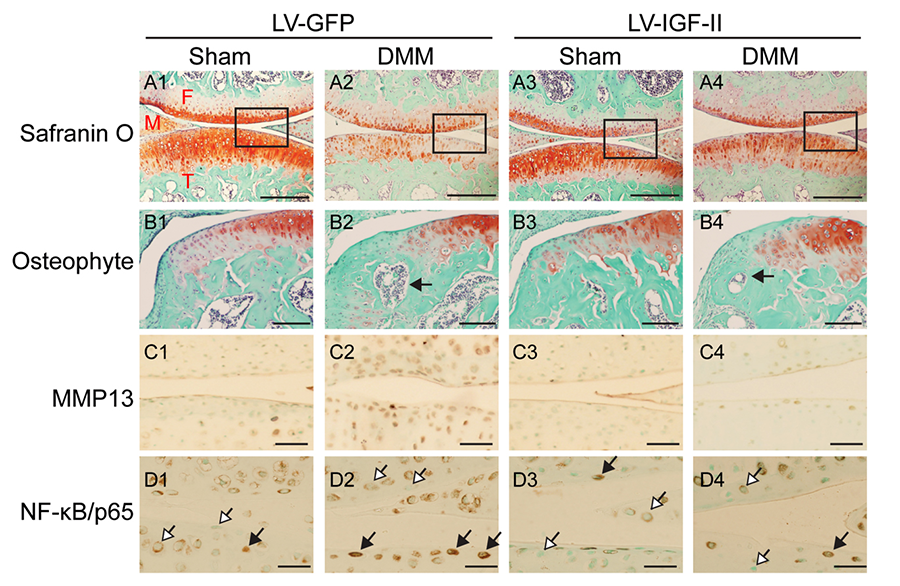
While most of the cartilage during development is replaced by bone, cartilage remains in certain locations in adults, including the articular surfaces of the bone and the intervertebral discs. Arthritis is characterized by the destruction of joint cartilage as well as pathological changes in other joint tissues. Inflammation plays a critical role in the initiation and exacerbation of joint degeneration. The most common form of arthritis is osteoarthritis (OA). We have been studying regulators of OA development using mouse OA models (including the destabilization of the medial meniscus (DMM) and the monosodium iodoacetate models), as well as using human OA cartilage specimens. In one study, we found that IGF2 inhibits pro-inflammatory cytokine-induced matrix loss in chondrocytes, and intrarticular injection of lentiviral IGF2 could halt OA progression (Uchimura et al, 2015; Fig. 4).
Figure 4. IGF-II promotes joint cartilage integrity in mouse experimental osteoarthritis. Shown here are sagittal sections of the knee joint, where femur (F), tibia (T) and meniscus (M) are indicated (Uchimura et al, 2015)
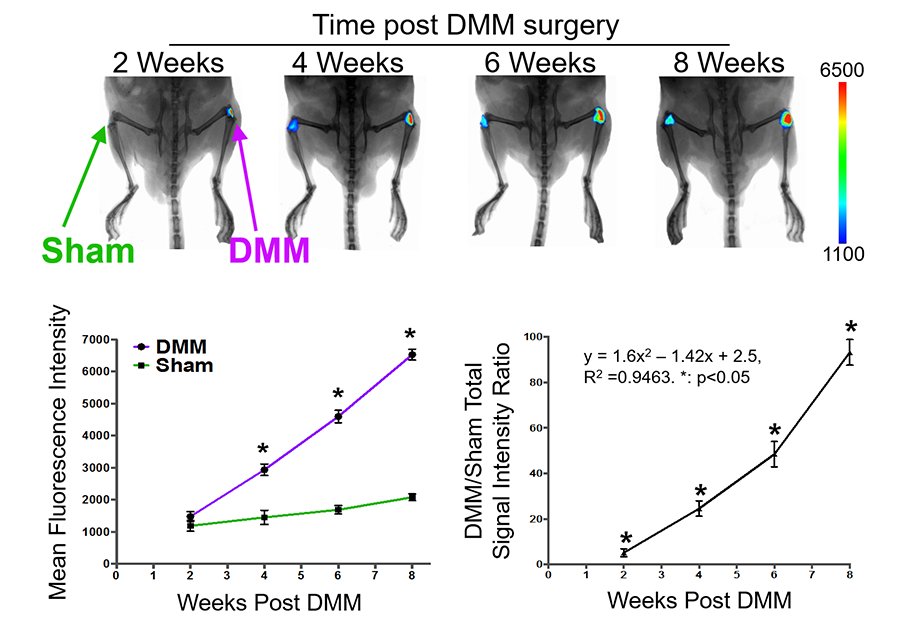
We are also exploring enabling technologies to facilitate our study. A common technological barrier for OA investigation is the lack of methods that allow for tracking of joint destruction over the course of joint degeneration, as opposed to single time point analysis, which slows down the pace of drug discovery. One technology is near infrared fluorescence (NIRF) imaging. Specifically, we have used a synthetic substrate of various MMPs (including MMP1, 13 and 9), which emits near infrared fluorescence when cleaved, therefore allowing us to follow MMP activities in vivo (Leahy et al, 2015). Our study showed that this probe exhibited progressively increased fluorescence in experimental mouse OA knees as compared to the sham, reflecting the trajectory of joint degeneration over time in the OA (Fig. 5).
Figure 5. Near Infrared Fluorescence (NIRF) imaging using an MMP activatable probe. Continued increase in NIRF signals were observed during mouse OA development (Leahy et al, 2015).
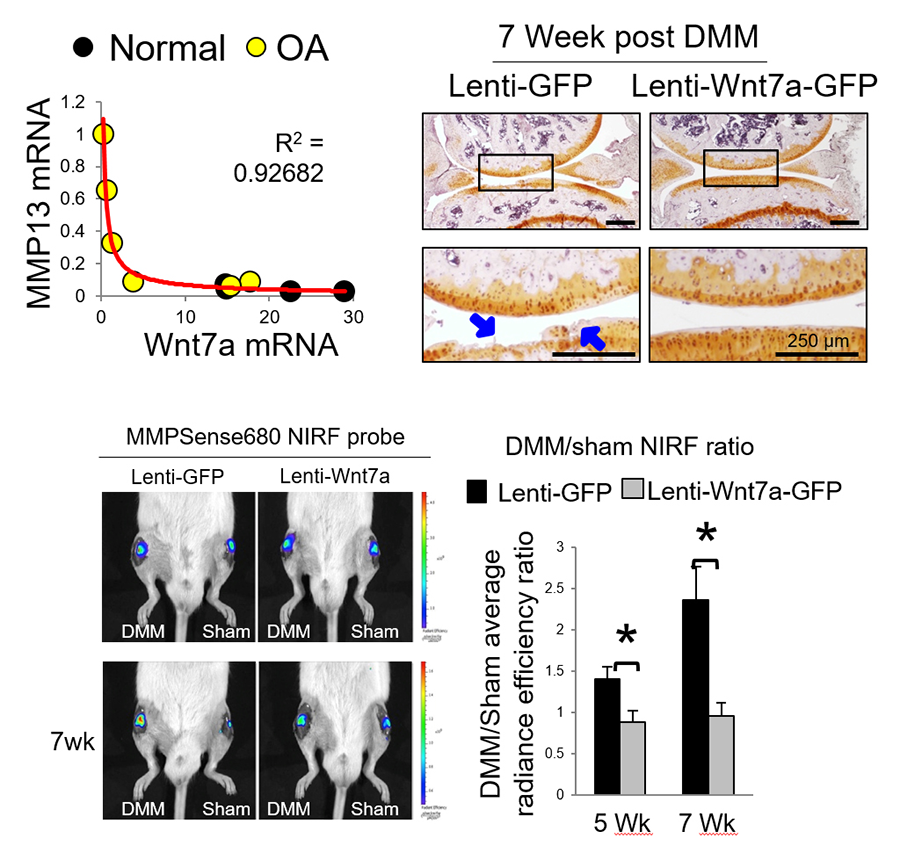
NIRF imaging has been used in our study of Wnt7a, which we found to promote joint integrity and inhibit the trajectory of OA (Fig. 6) (Gibson et al, 2017).
Figure 6. NIRF imaging indicates Wnt7a inhibits the trajectory of OA development (Gibson et al, 2017).
Cartilage Regeneration
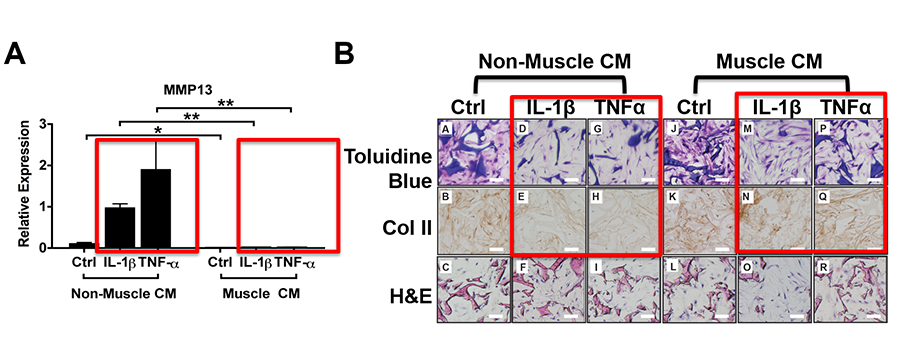
We are developing novel strategies to integrate concepts and approaches from developmental biology studies with tissue engineering, in order to engineer stronger and more stable cartilage tissue for repair. In collaboration with Dr. David Kaplan from the Department of Biomedical Engineering, our laboratory discovered that muscle cell-expressing factors have the capacity to promote matrix production in engineered cartilage of three-dimensional cultures and resist pro-inflammatory cytokine-induced matrix loss (Rainbow et al, 2013). We also examined the impact of different scaffolds on the stability of engineered cartilage under inflammatory conditions. In particular, we discovered that scaffolding materials and structure influence the behavior of chondrocytes by influencing the microenvironment, and should be considered as an important component for bioengineering stable cartilage tissues (Kwon et al, 2013, Kwon et al, 2015; Fig. 7).
Figure 7. Muscle cells-secreted factors promote cartilage matrix expression under inflammatory conditions in chondrocytes cultured in 3D silk scaffolds (Rainbow et al, 2013).