The Mercio Perrin Lab
Deciphering Mechanisms Underlying Chagas Disease Progression
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi and affects millions of people worldwide, including the United States. The disease progresses through acute and chronic phases. Most patients (~99%) survive acute infection despite tissue damage, particularly in the heart, caused, to a great extent, by robust tissue parasitism and corresponding inflammation. Parasite growth is kept in check by the immune system while cardiac function is restored by endogenous repair mechanisms. Conversely, chronic infection may present severe abnormalities in the heart (cardiomyopathy) and gastrointestinal track (megaesophagus and/or megacolon) years after infection. Here, pathology is progressive and irreversible, often a cause of death. The reason for these distinct outcomes is poorly understood. To gain insights into mechanisms governing in Chagas disease progression we focus on two areas described below.
T. cruzi transendothelial migration
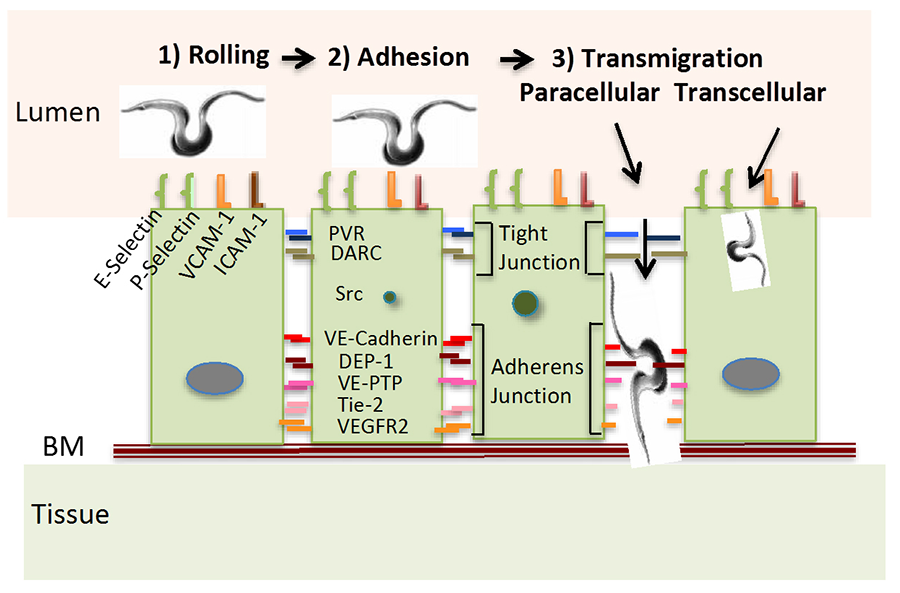
Leukocyte recruitment, recirculation and trafficking require adhesion and transmigration through the vascular endothelium, which starts with leukocyte binding to adhesion molecules (selectins, ICAM-1 and VCAM-1) located on the apical surface of the endothelium on post-capillary venules. This reduces the speed of free-flowing leukocytes (rolling) to allow adherence to the endothelial surface. Crossing the endothelium is via paracellular and transcellular routes that require additional adhesion molecules, and receptor and nonreceptor kinases and phosphatases on endothelial cell junctions. Like leukocytes, T. cruzi must traverse the endothelium to thrive in targeted organs, where they enter cardiomyocytes, fibroblasts and other types of cells to grow and differentiate, a process essential for parasite survival in mammalian hosts. However, the molecular basis governing such vital process in the parasite life cycle remains a mystery.
We hypothesize that T. cruzi takes advantage of at least some of the endothelial molecules required for leukocyte transendothelial migration (see Fig. 1). The model is based on transmigration genes used by leukocytes whose expression is altered by T. cruzi following a pattern compatible with that by host immune cells (and cancer cells). Additionally, we have identified a T. cruzi surface molecule that mimics the actions of the parasite, which will facilitate understanding the molecular basis of T. cruzi-endothelial cell interaction.
Figure 1. Model of T. cruzi Transmigration Through the Endothelium The model is a replica of current concept of leukocyte and cancer cell extravasation into the parenchyma of tissues; BM, basement membrane; VCAM-1, vascular cell adhesion molecule-1; ICAM 1, intercellular adhesion molecule 1; PVR, poliovirus receptor; DARC, Duffy antigen receptor for chemokines; DEP-1, density-enhanced phosphatase 1; VE-PTP, vascular endothelial phosphotyrosine phosphatase; Tie-2, tunica interna endothelial cell kinase, a.k.a. angiopoietin-1 receptor; VEGFR2, vascular endothelial growth factor receptor 2; BM, basement membrane.
In addition to revealing how T. cruzi interaction with the endothelium leads to extravasation organs required for the intracellular cycle of the parasite, this may also unveil agents that interfere transmigration, tantamount to antitrypanosomal drugs. It is also possible the project will uncover ways to interfere with leukocyte transmigration such as by understanding why T. cruzi can pass through endothelium of uninflamed organs (such as during first-time invasion) as opposed to leukocytes that readily can cross endothelium only in injured and inflamed tissues.
Mutually beneficial T. cruzi helps in infected-tissues repair
It is unclear why effective cardiac regeneration prevails in acute Chagas disease, in which parasites abound, and not in chronic cardiomyopathy, in which parasites are rare. This paradox could be reconciled if T. cruzi were able to help the host repair infected and wounded cardiac tissues. We have identified a T. cruzi surface protein, PDNF (parasite-derived neurotrophic factor) that mimics the neurotrophins NGF (nerve growth factor) and NT-3 (neutrotropin-3). PDNF activates survival-signaling pathways (Akt and MAP kinases) and protects cells of the nervous system and heart against damaging insults such oxidative stress.
Testing the hypothesis that PDNF is responsible for repair in acute infection would be straightforward using T. cruzi genetically deficient in PDNF. This approach is not practical because T. cruzi has more than one thousand copies of the PDNF gene. A corollary of the hypothesis is that chronic cardiomyopathy progresses, in part, by the paucity of T. cruzi and logically of survival factors such as PDNF. Therefore, systemic administration of PDNF in mice bearing chronic Chagas cardiomyopathy (CCC) should increase levels of PDNF in tissues to approach those at acute infection. If our hypothesis is valid, than systemic PDNF administration is expected to mitigate heart pathology in CCC mice. This is verified experimentally using genetic, biochemical, immunocytochemistry, echocardiography, and other approaches.
We are testing how PDNF protects the heart at the cellular and molecular levels, specifically, identifying the cardiac cells T. cruzi PDNF reacts with in CCC, genes altered, up- and down-regulated paracrine and autocrine signaling. It is apparent that this hypothesis has therapeutic implications not only for the treatment of CCC but also for cardiac diseases distinct from Chagas disease.