The Mary Wallingford Lab
Placental Vascular Development and Pathophysiology
The overarching research objective of the Wallingford Lab is to advance fundamental knowledge of placental vascular development and pathophysiology and to advance obstetric cardiovascular medicine by supporting the development of diagnostic tools and treatment options for placental disorders.
Our focus is on morphogenetic mechanisms that control vascular development and pathophysiology of the least understood organ: the placenta. Normal growth and function of the placenta are absolutely essential for maternal and fetal health, both during pregnancy and later in life. Placental dysfunction can lead to preterm birth, preeclampsia (a hypertensive disorder caused by placental dysfunction), and loss of life. Mothers who have had preeclampsia are at increased risk for cardiovascular disease and coronary artery calcification. Babies who were exposed to preeclampsia are more likely to develop hypertension and increased BMI into their teenage years, and they have an increased stroke risk later in life. Currently, there are few diagnostic tools and no curative therapeutic options for preeclampsia besides immediate delivery of the placenta, often necessitating preterm delivery. The dearth of early diagnostics and treatments stems in part from a lack of understanding of how placenta and placental diseases develop.
Morphogenetic Analysis of Placentation
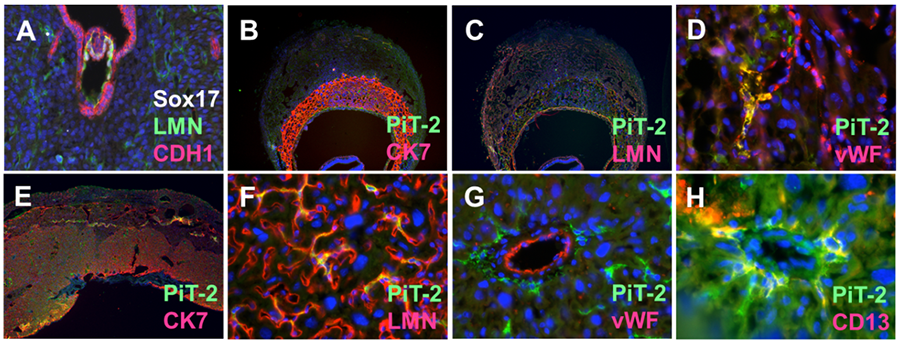
Our Morphogenetic Analysis of Placentation (MAP) project investigates developmental mechanisms that control placental blood vessel formation, vascular remodeling, and vascular mimicry. Building on early placentation research that Dr. Wallingford conducted in the Mager Lab (Wallingford et al, 2013), the MAP group applies vascular morphometric and cellular phenotyping to developmental models and tractable 3D culture systems in order to determine how blood vessel formation, vascular remodeling, and vascular mimicry are controlled at the molecular level. Dynamic vascular remodeling of the mouse placenta is well exemplified by examination of proteins that identify trophoblasts, vascular cells, and tissue structures (Figure 1).
Figure 1. Vascular Remodeling in Mouse Placentation. A. Implanting mouse embryo at E4.0. Laminin (LMN) highlights vascular structures in the decidua and E-Cadherin (CDH1) highlights the breakdown of uterine luminal epithelium. B-C. E9.5 Mouse placenta labelled with antibodies against Slc20a2, trophoblast cytokeratin 7 (CK7), LMN and endothelial von Willebrand’s Factor (vWF) localization. E-H. E15.5 placenta with PiT-2, CK7, LMN, vWF, and pericyte (CD13) localization.
In addition to in vivo studies, we use 3D cell culture approaches to investigate how vascular tissue and specialized niches develop and function. As we expand our placenta research tool set, the MAP group lab would gladly serve as a unique resource to local investigators who wish to expand into collaborative placenta research.
Maternal-Fetal Phosphate Transport
Phosphorus is an essential nutrient that plays numerous roles in human health. It is a primary component of the mineral that bones are made of, yet it also performs many other important functions and disrupted phosphate homeostasis can lead to adverse pathologies including vascular calcification. While a large body of scientific research describes phosphate biochemistry in the adult, very little is known about how circulating inorganic phosphate gets to the developing baby.
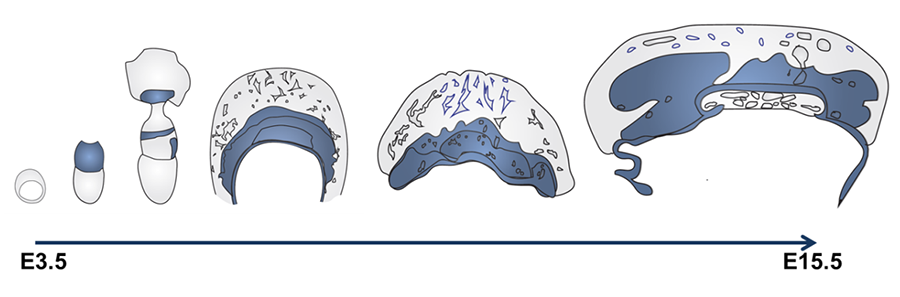
Our Maternal-Fetal Phosphate Transport (M-FPT) project is closing this knowledge gap by defining maternal-fetal phosphate transport routes, testing hypotheses on the basic science of placental phosphate transport, and developing new biological assays for maternal-fetal phosphate homeostasis that may one day be useful for assessment and interpretation of maternal and fetal health in the clinic. This work has identified a critical role for the placental sodium-dependent phosphate transporter Slc20a2 in fetal growth and development as well as protection from placental vascular calcification (Wallingford et al, 2016; Yamada & Wallingford et al, 2018a). Slc20a2 expression domains in the mouse placenta (Figure 2) correspond with vascular abnormality and ectopic mineralization domains observed in Slc20a2-depleted placenta, providing the first genetic link and animal model for placental calcification.
Figure 2. Slc20a2 is expressed at the mouse maternal-fetal interface. Regional Slc20a2 expression during mouse placentation is indicated in blue. Slc20a2 expression detected by beta-galactosidase staining of Slc20a2-driven LacZ was observed in the ectoplacental cone, chorion, allantois, parietal endoderm, fetal endothelial cells, trophoblasts, and pericytes.
Clinically, calcification has been observed in placenta, but the biomedical significance of placental calcification is poorly understood. The relationship between placental calcification and hemodynamic alterations in conditions such as preeclampsia, placental thrombosis, and intrauterine growth restriction remains unknown. These unknowns are discussed in detail in a recent publication from our lab that reports on novel microcalcification patterns in human placenta (Wallingford et al, 2018b).
In future work the M-FPT research group will 1) determine placental phosphate transport mechanisms in extraembryonic tissue (NIH R00HD090198) and 2) evaluate the biomedical significance and prognostic value of placental calcification (AHA 19CDA34660038).