The Miaofen Hu Lab
Mouse Models of CDK6 Function
Cyclin-dependent kinases (CDKs) are serine/threonine kinases whose activity relies on a regulatory subunit - a cyclin. Human cells have 20 CDKs and 29 cyclins. However, only certain CDKs are directly involved in driving the cell cycle. Cell cycle-related CDKs are further divided into three interphase CDKs (CDK2, CDK4, and CDK6) and one mitotic CDK (CDK1), which are required for cell cycle progression. The key regulator of G1-S transition is the cyclin D-CDK4/6-INK4-pRB pathway.
Since their discovery, CDK4/6 have been widely thought to be important for initiation of the cell cycle in response to growth regulatory signals and to act redundantly to regulate the cell cycle progression through the phosphorylation of pRB. Although CDK4 and CDK6 share 71% amino acid identity and have largely overlapping functions, it has become clear that preferential expression of CDK4 or CDK6 in some tissues supports the idea that these kinase subunits may have distinct functions. For instance, CDK4 is highly expressed in mesenchymal cell types such as fibroblasts and osteoblasts, whereas CDK6 is predominant in all hematopoietic cell types.
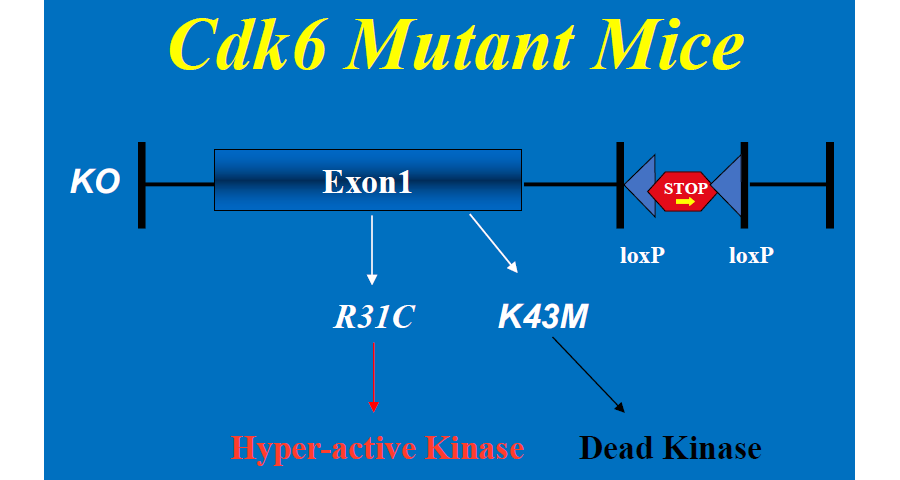
To address the role of CDK6 in development and tumorigenesis, we have produced both knockout (Cdk6-/- or KO) and knock-in mice (Figure 1) by introducing a LoxP-flanked transcriptional STOP cassette into intron 1 of the Cdk6 gene adjacent to the intact /mutant exon 1. In the presence of the STOP cassette CDK6 expression is prevented, resulting in a null allele (Cdk6–/– or KO). Upon excision of the cassette by CRE recombinase, the CRE-reactivated wild-type allele or the mutant alleles express WT or mutant CDK6, respectively, from the endogenous locus with intact regulatory controls. The knock-in mutants include CDK6R31C (R31C), a hyper-active, inhibitor-resistant kinase that cannot interact with INK4 family inhibitor proteins such as p16, and a catalytically inactive kinase, CDK6K43M (K43M). The R31C mutant mimics hyperactivation of CDK6 in tumor/disease cells, whereas the catalytic inactive K43M mutant model pharmacological inhibition of kinase activity. By using our Cdk6 mouse models, we have been studying the role of CDK6 in T-cell acute lymphoblastic leukemia (T-ALL) and obesity/obesity related diseases.
Figure 1. Cdk6 mutant mice.
CDK6 as a Novel Therapeutic Target for T-ALL/LBL
T-cell acute lymphoblastic leukemia/T-cell lymphoblastic lymphoma (T-ALL/LBL) is a cancer of immature T-lymphocytes that appears to arise from an early bone marrow-derived precursor. More than half of T-ALLs have activating NOTCH1 mutations or abnormalities in the PTEN-AKT pathways. Complex combination chemotherapy is generally effective at inducing remission of the disease. However, a high proportion of T-ALL patients suffer relapse, possibly because the available therapies do not eradicate leukemic stem cells (LSCs) that initiate and sustain the disease. Small-molecule gamma-secretase inhibitors (GSIs), which block a critical proteolytic step required for NOTCH1 activation, have activity against T-ALLs with NOTCH1 mutations but not those with PTEN deficiency or constitutively active AKT. The clinical development of GSIs in T-ALL has been hampered by gastrointestinal toxicity, while therapeutic responses to GSIs are modest and transient. In addition to acquired loss-of-function mutations in PTEN, GSI resistance may also develop in a small subset of primary T-ALL cells through BRD4-dependent epigenetic chromatin modifications that sustain expression of several NOTCH target genes, including MYC, BCL2, and CDK6.
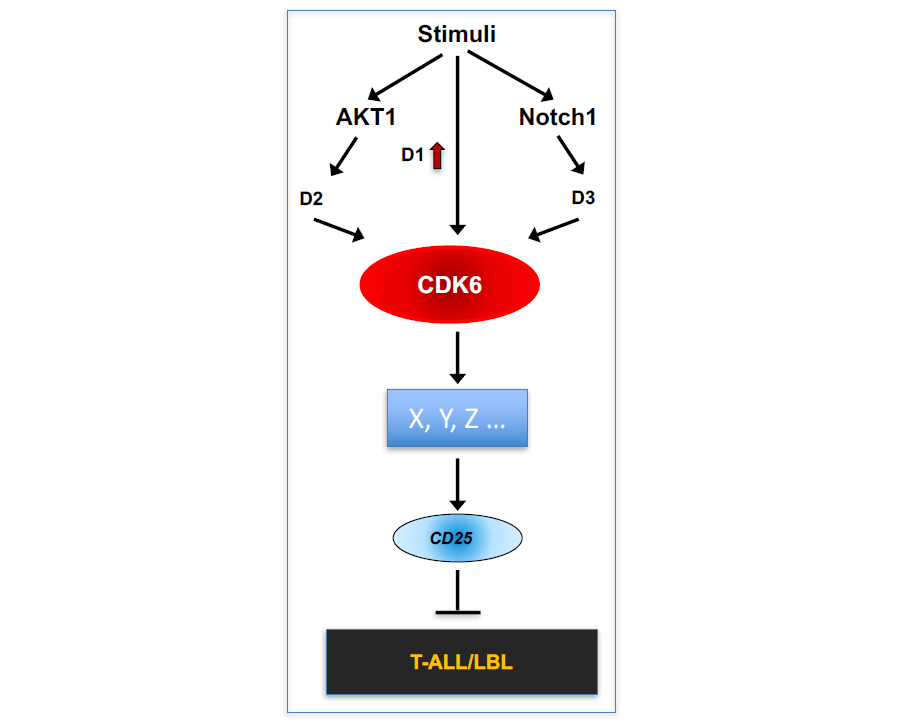
Figure 2. A working model of the role of CDK6 in T-ALL/LBL.
Employing Cdk6 mutant mice, we have found CDK6 serves a common downstream effector of both AKT1 and Notch1 signaling pathways (Fig. 2). We have also found that CDK6-mediated suppression of CD25, the α-chain of IL2R, is required for initiation of T-ALL by activated Notch1, and CD25 induction mediates the therapeutic response to CDK6 inhibition in established T-ALL. Those evidence leads to a novel hypothesis that CDK6 plays an essential role in human T-ALL formation and maintenance, and that inhibition of CDK6 leads to therapeutics for this subset of T-ALL and CD25 expression could serve as a biomarker for responsiveness of T-ALL to CDK6 inhibitor therapy.
Now, we are actively pursuing the mechanism involved in the regulation of CD25 by CDK6 in mouse and in human T-ALL/LBL cells.
CDK6 as a novel therapeutic target for obesity and its related metabolic diseases
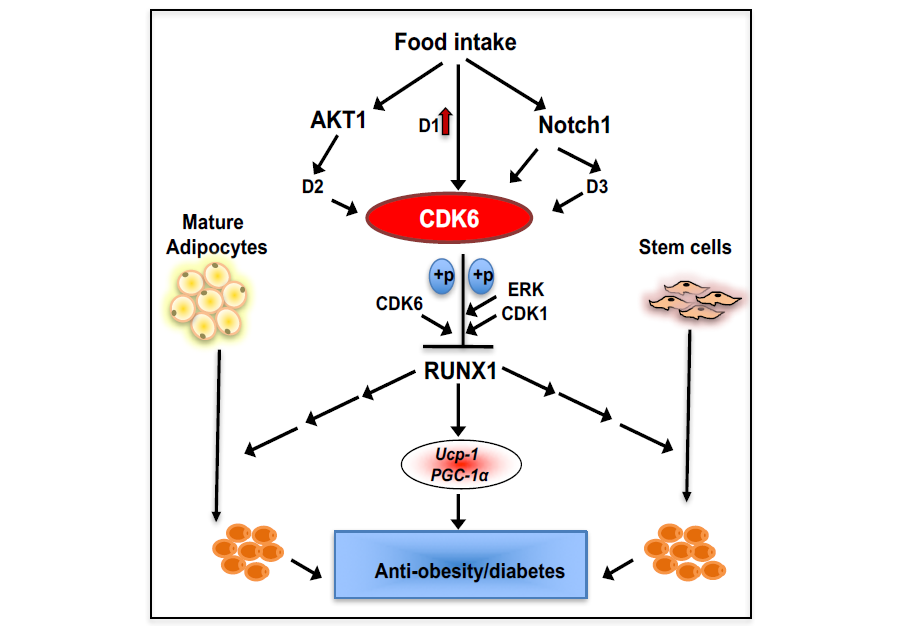
Whereas white adipose tissue depots contribute to the development metabolic diseases, brown and beige adipose tissue has beneficial metabolic effects. Using our Cdk6 mouse models, we show (Fig. 3) that CDK6 regulates beige adipocyte formation. We demonstrate that mice lacking the CDK6 protein or its kinase domain (K43M) exhibit significant increases beige cell formation, enhanced energy expenditure, better glucose tolerance and improved insulin sensitivity, and are more resistant to high fat diet–induced obesity. Re-expression of CDK6 in Cdk6-/- mature or precursor cells, or ablation of RUNX1 in K43M mature or precursor cells, reverses these phenotypes. Furthermore, RUNX1 positively regulates the expression of Ucp-1 and Pgc-1α by binding to proximal promoter regions. Our findings indicate that CDK6 kinase activity negatively regulates the conversion of fat-storing cells into fat-burning cells by suppressing RUNX1, and suggest that CDK6 may be a therapeutic target for the treatment of obesity and related metabolic diseases.
Figure 3. A working model of the role of CDK6 in white fat browning.
We have also found that loss of CDK6 protein or kinase activity (K43M) in mice results in reduced fat mass in visceral adipose tissues (VAT) but not through negative regulation of white fat browning. Moreover, ablation of RUNX1 in K43M mice reverse the phenotypes observed in K43M mice, which is also independent of negative regulation of white fat browning in VAT. Thus, it is unknown at present how CDK6 affect the biology and metabolism of VAT, which is of particular concern because it is linked to metabolic dysfunction and increased risk for heart diseases and non-insulin dependent diabetes - much more so than SAT. It is also unknown at present if we can potentially translate our finding in mice to human patients.