The Emmanuel Pothos Lab
Mechanisms of Synaptic Neurotransmission in Central Monoamine Systems
Our laboratory studies cellular and molecular mechanisms of synaptic neurotransmission in central monoamine systems. We mostly focus on CNS catecholamines because of our interest in the neurochemistry and molecular biology of catecholamine-related disorders. Specific objectives include synaptic plasticity in CNS catecholamine systems as it relates to normal function and the pathogenesis of dietary obesity, drug and high-energy food addiction, and neurodegenerative disorders.
Presynaptic mechanisms of quantal size modulation in midbrain dopamine neurons
Quantal size is defined as the number of neurotransmitter molecules released by a single synaptic vesicle during exocytosis. Contrary to conventional assumptions, we believe and we have shown that when measured accurately (that is from the presynaptic site by amperometric carbon fiber electrodes), dopamine quantal size is not fixed and can be pharmacologically manipulated. This approach allowed the first ever presynaptic observation of CNS quanta, and provided the number of molecules and the duration of release during exocytosis (3,000-14,000 molecules over 200 microseconds under control conditions; Pothos et al., 1998; Pothos and Sulzer, 1998; Sulzer and Pothos, 2000; Pothos, 2002).
Modulation of Quantal Parameters
Based on the discovery that quantal size can exhibit remarkable plasticity, we have introduced the hypothesis that changes in catecholamine release may be due to altered quantal size (and not only due to different number of vesicles being released). This hypothesis runs contrary to the principle of the invariability of quanta, as originally introduced by the work of Bernard Katz and colleagues. We have worked on the identification of several mechanisms that modulate quantal size in mesolimbic terminals:
Regulation of Quantal Size by Activity and Neurotransmitter Synthesis
A form of long-term potentiation (LTP) at mesolimbic synapses may be elicited by protein kinase A (PKA) activation. LTP refers to long-term facilitation of neurotransmitter release at a given synapse following repeated stimulation. In catecholamine synapses, PKA may induce LTP by increasing neurotransmitter quantal size through phosphorylation of tyrosine hydroxylase (TH) and, therefore, through changes in neurotransmitter synthesis. An increase in dopamine synthesis (via l-DOPA) has been previously shown to increase quantal size in both PC12 cells (Pothos et al, 1996) and neurons (Pothos et al, 1998b). A further mechanism of activity-dependent altered quantal size is provided by stimulation-dependent acidification of the vesicles (Pothos et al., 2002), allowing greater vesicular transmitter uptake. In chromaffin cells, we found that depolarization further acidifies granule pH in tandem with elevated quantal size. This may be due to depolarization-dependent phosphorylation of a vesicular chloride channel, which causes changes in the vesicular proton gradient.
Regulation of Quantal Size by the Neuronal Vesicular Monoamine Transporter (VMAT2)
Although vesicular accumulation of transmitter is thought to reach a steady state based on the electrochemical gradient, it is possible that expression of vesicle transporters provides regulatory steps. To examine this, we used mice with disrupted genes for VMAT2, the brain vesicular monoamine transporter. Underexpression of the transporter reduced vesicular transmitter storage (Fon et al., 1997). To examine transporter overexpression, we used both midbrain neurons and secretory cell lines (AtT-20 and PC12 cells) exposed to an adenovirus construct. VMAT2 overexpression resulted in a 4-fold increase in both quantal size and the number of vesicles released per stimulation (Pothos et al., 2000).
Regulation of Quantal Size by Neurotrophic Factors
Growth factors acting as retrograde messengers may also modulate presynaptic mechanisms. We established that GDNF increases quantal size and release frequency of midbrain dopamine neurons by nearly 5-fold, an effect that lasts at least one month after exposure (Pothos et al., 1998a). We are further planning to identify and study the proteins involved in these effects.
Regulation of Quantal Size by Psychostimulants and Other Drugs of Abuse
Reuptake blockers such as cocaine and amfonelic acid significantly reduced dopamine quantal size in a D2-independent manner (Pothos et al, 1998c), suggesting that reuptake blockade directly affects neurotransmitter storage by reducing cytosolic transmitter levels. This mechanism differs from amphetamine-induced reductions in quantal size, which stem from the collapse of the vesicular pH gradient and release of dopamine into the extracellular space by reverse transport.
Drug Dependence
We published the first studies to demonstrate the involvement of mesoaccumbens dopamine and acetylcholine in opiate withdrawal (Pothos et al, 1991; Rada et al, 1991). Rats undergoing morphine withdrawal (precipitated by naloxone) showed depressed basal extracellular dopamine and elevated acetylcholine in the nucleus accumbens, but both effects were abolished after clonidine treatment.
Central Dopamine, Obesity and Obesity resistance
The concept of homeostasis and regulation of body weight through hypothalamic neurohormonal circuits have traditionally dominated the obesity research field. The recent transgenerational explosion of dietary obesity in Western societies clearly shows that this approach is inadequate as homeostatic mechanisms, if dominant, would not have allowed such a worldwide and overwhelming increase in body mass index. There is a need to examine why so many patients fail to follow the simple rule of energy balance (if caloric intake equals energy expenditure there is no net body weight gain). Our approach places emphasis on those CNS circuits that code for food reward, the mesolimbic dopamine systems. An important result in this area of studies is our finding that dopamine release is depressed by 60% in the nucleus accumbens of rats kept on a high-fat diet for several weeks with a consequent weight gain of at least 20% (Pothos et al., 1998d). A challenge with regular laboratory chow showed a decreased dopamine release in the overweight animals, but a challenge with the high-fat food showed a significant dopamine increase (Geiger et al., 2009). Furthermore, when we compared neonatal rats inbred to show obesity proneness or obesity resistance, we found that the number of dopamine transporters (DAT and VMAT2) and dopamine quantal size are higher in the obesity-resistant group (Figure 2; Geiger et al., 2008 ). Therefore, even without any diet history, genetic predisposition to obesity is characterized by depression of mesolimbic dopamine neurotransmission at a very early age; while genetic predisposition to obesity resistance is characterized by upregulation of mesolimbic dopamine neurotransmission.
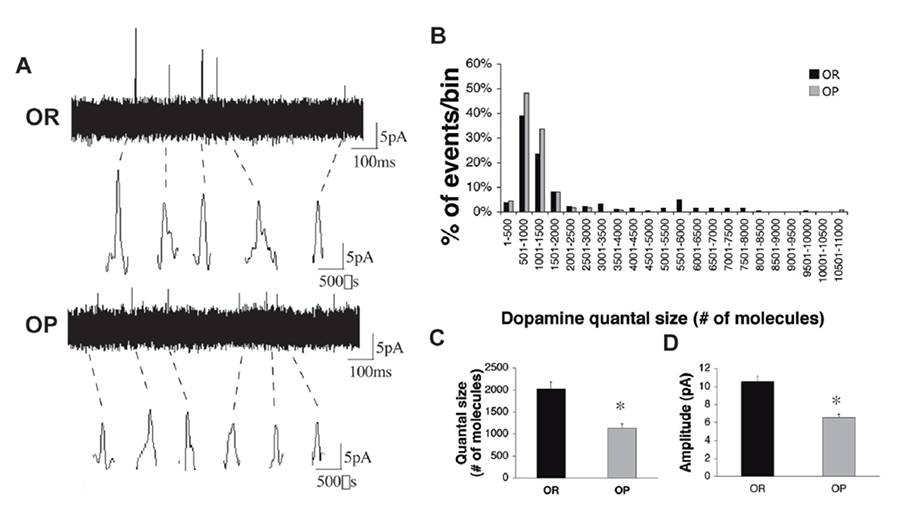
Figure 1. Reduced dopamine quantal size in VTA-derived neurons from P0-P1 obesity-prone pups. (a) Representative amperometric traces from VTA cultures of obesity-resistant (top) and obesity-prone (bottom) neonates. Individual events are shown at higher resolution below. (b) Quantal size distribution in cultures from neonatal obesity-prone and obesity-resistant animals. Note the obesity-prone distribution is skewed to the left due to lack of events in the higher quantal size bins. (c-d) Quantal size and amplitude of stimulated dopamine release from VTA-derived neuronal cultures from obesity-prone pups (grey bars, n=110 events) is lower than in cultures derived from obesity resistant pups (black bars, n=182 events). *p<0.01 (Geiger et al, 2008).
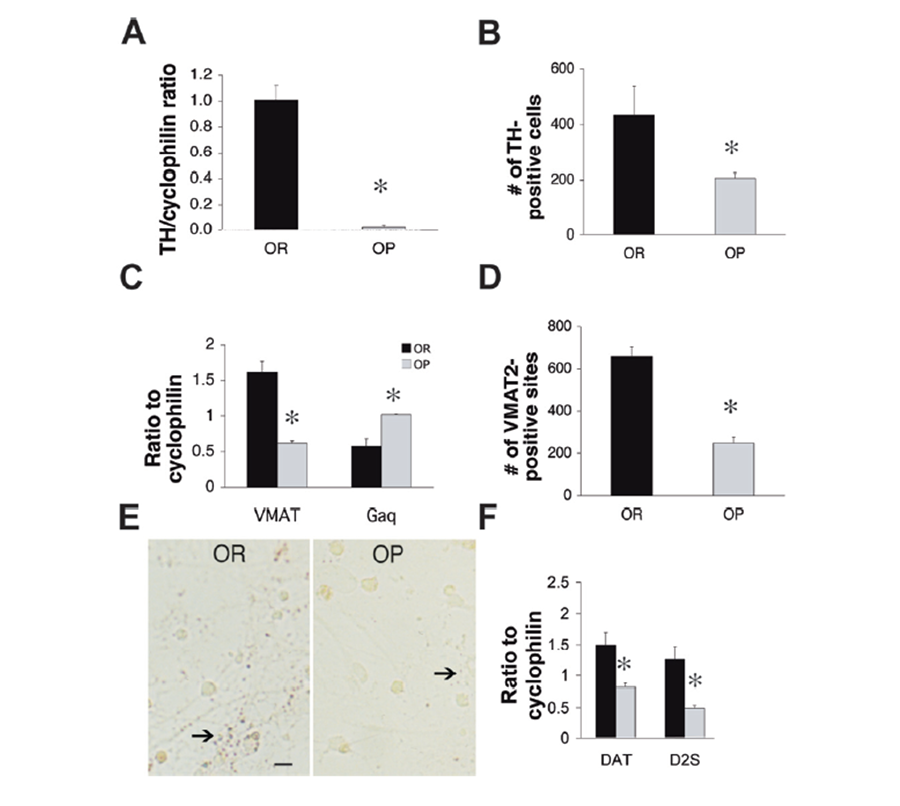
Figure 2. Lower mRNA and protein expression regulators of dopamine synthesis and exocytosis VTA cell cultures from obesity-prone neonatal animals. (a) mRNA expression of TH was significantly lower in dopamine neuronal cultures derived from obesity-prone rats (grey bar) than in those from obesity-resistant rats (black bar, n=5 pooled cultures/group in triplicate). (b) TH immunopositive cells in cultures from obesity-prone rats (n=11 cultures) were significantly less than in those from obesity-resistant rats (n=6 cultures). (c) mRNA expression of VMAT2 was significantly lower while mRNA expression of endogenous VMAT2 downregulator Gαq was significantly higher in cultures from obesity-prone rats than in those from obesity-resistant rats (n=5 cultures/group). (d) VMAT2 immunopositive sites in cultures from obesity-prone rats (n=12 cultures) were significantly less than in those from obesity-resistant rats (n=16 cultures). (e) Representative VMAT2 immunostaining in VTA cultures from obesity-resistant and obesity-prone pups, 7 days post plating. Arrows point to VMAT2-positive sites. Scale bar=100µm; 20x magnification. (f) Lower mRNA expression of the DAT transporter and the presynaptic dopamine autoreceptor D2S in cultures from obesity-prone (grey bars) than obesity-resistant neonates (black bars, n=5 cultures/group). *p<0.01 (Geiger et al, 2008).
The above findings also apply to genetic models of obesity and obesity resistance in the mouse. The ob/ob leptin knockout mouse and the melanin concentrating hormone (MCH) knockout mouse are the two preparations that we focus on as they convincingly demonstrate an obese and a diet-resistant phenotype respectively. We have published that dopamine signaling in the ob/ob mouse is severely compromised (Fulton et al., 2006). This series of studies has identified previously unknown and unappreciated CNS markers of obesity predisposition and obesity resistance and points to new directions for the treatment of the obesity epidemic as it addresses central mechanisms of food reward as key components of the neurochemistry of obesity. These mechanisms should constitute targets for therapeutic intervention.
Parkinson's Disease
Beyond the study of the mechanism of action of drugs like levodopa, our research effort is now expanding into the examination of possible genetic substrates of the disease. Recent clinical studies have identified parkin, DJ-1 PINK1 and LRRK2 gene mutations as closely associated with familial Parkinson's disease (eg Shen and Cookson, 2004). Through an extensive collaboration with the Harvard Neurology Group at Brigham and Women's Hospital, we have initiated a series of studies with parkin, DJ-1 and PINK1 deficient mice in order to assess their behavioral, neuroanatomical and neurochemical phenotype and evaluate the role of these gene products in the onset and development of the disease. Our findings so far suggest that targeted deletion of the parkin, DJ-1 and PINK1 genes or overexpression of the LRRK2 gene leads to defects in locomotor behavior and/or stimulated dopamine release consistent with the phenotype of Parkinson's Disease (Goldberg et al, 2005; ; Kitada et al, 2007; Tong et al, 2009).
Magnetic Field Excitation
In collaboration with the Tufts Department of Computer and Electrical Engineering and colleagues inside and outside Tufts, we have initiated a study on the effects of magnetic excitation on neurotransmitter release and cell death. The objective is to identify the biological substrates involved in cell death following exposure to magnetic fields similar to those emitted by handheld electronic devices (i.e. cell phones and PDAs) and household appliances (i.e. microwave ovens). Furthermore, this line of research will seek to identify the substrates involved in the deep magnetic stimulation therapy currently used for treatment of Parkinson’s disease, depression and psychotic disorders.
| Ion | Earth Magnetic Induction | |||
| 30μT | 50μT | 60μT | 70μT | |
| Li+ | 66.37 | 110.61 | 132.74 | 154.85 |
| Na+ | 20.04 | 33.4 | 40.08 | 46.75 |
| Mg2+ | 37.9 | 63.16 | 75.8 | 88.43 |
| Cl- | 13.37 | 22.29 | 26.74 | 31.2 |
| K+ | 11.78 | 19.64 | 23.56 | 27.49 |
| Sr2+ | 10.51 | 17.52 | 21.02 | 24.53 |
| Ca2+ | 22.99 | 38.31 | 45.98 | 53.64 |
Table 1. Cyclotron resonance frequency in Hz for the most significant ions for neurotransmitter exocytosis. 60 µT is the earth’s magnetic induction in Boston, MA and the frequencies on that column would apply for the magnetic fields studied here.
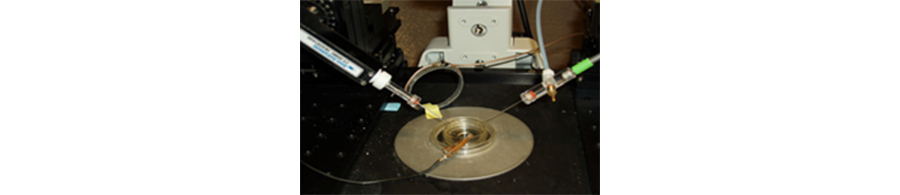
Figure 3. The circular magnetic coil (center left) used on the microscope stage to induce exposure to magnetic fields. On the left is the recording carbon fiber microelectrode and on the right the stimulating solution glass pipette. The 35mm dish with the cell culture and the ground electrode are pictured in the center. The current flow through the coil is controlled by a computer-driven mini-amplifier.
We have now preliminary evidence to suggest that the cyclotron resonance frequency specific for Ca2+ ions (44-48 Hz, table 1) is the one to significantly increase neurotransmitter quantal size, alter properties of Ca2+ channels, increase Ca2+ entry into the cytosol and, therefore, likely to induce cell death. We accordingly hypothesize that exposure to calcium-specific cyclotron resonance frequencies can potentiate biogenic amine neurotransmitter exocytosis and induce deleterious effects on paracrine and neuronal cells that may suffer damage from prolonged exposure to products of the excess neurotransmitter oxidation. If confirmed, this finding can potentially lead to fundamental innovations in the design of electronic devices so that harmful effects of electromagnetic emissions can be blocked, while beneficial effects of magnetic brain stimulation therapy used in clinical settings are maximized.