The Shruti Sharma Lab
The hallmark of autoimmunity is the dramatic loss of immune homeostasis. Early work in autoimmune models identified the role of various innate pathways, such as nucleic acid sensing TLRs (TLR7 and TLR9), as direct contributors to disease. However, work in the MRL/lpr, TMPD-induced and Yaa models has revealed a complex relationship between TLR7 and TLR9 during chronic disease, pinpointing TLR9 instead as a negative regulator and TLR7 as a positive regulator of autoimmune pathology.
It is important to note that mechanistic understanding of TLR7 and TLR9 cross-regulation is still lacking. Similar to the initially described roles for TLR7/9 in autoimmunity, recent work has implicated cytosolic DNA sensing pathways mediated by the ER-resident protein STING, in directly promoting pathology during autoimmunity. However, using some of the above models, our studies now show that this initial understanding of STING-dependent responses in autoimmunity is similarly incomplete.
We noted that in a spontaneous (MRL/lpr) or inducible (TMPD) model of autoimmunity (normally dependent upon TLR7/9 for pathology), the loss of STING leads to increased morbidity and mortality through a dramatic expansion of the autoantibody repertoire, myeloid compartment and concomitant inflammatory responses. Mechanistically, the temporal loss of negative regulators of signaling as well as an early loss of the secreted tolerogenic factor IDO-1 enhance TLR-dependent inflammation in the absence of STING. These studies are the first to show the existence of a critical regulatory loop between nucleic acid sensing receptors in the endosome and cytosol during chronic inflammation.
Combining these recent findings with what is known about TLR9 during autoimmunity, we believe that the circuitry that senses nucleic acids maintains immune-homeostasis by triggering both immune activation and tolerance pathways early during development. However, open questions remain: (a) what are the cell types involved? (b) what are the key signaling molecules downstream of STING/TLR9 that engage tolerogenic pathways? (c) what is the nature of tolerogenic pathways? (d) what is the natural ligand for engaging these ‘tonic’ pathways? (e) how do these pathways impact the immune system during homeostatic functions that regulate the fitness of the organisms, such as metabolism, steady-state hematopoiesis, aging etc.
The central goal of my work is to elucidate the mechanisms by which cell-intrinsic nucleic acid sensing pathways contribute to immunological tolerance and how their involvement in inflammatory or tolerogenic pathways shape the general fitness of the organism. We will achieve this by: mapping the epigenetic and proteomic circuitry of innate tolerogenic pathways downstream of nucleic acid sensing pathways; identifying the cell-types in which the nucleic acid sensing machinery directs a tolerogenic signature during innate immune responses; understanding the contribution of these pathways to metabolic fitness, development, aging and adaptive tolerance.
By systematically reconstructing the networks of tolerance in innate immune responses, we will be able to decipher cellular and molecular criteria by which tolerogenic and inflammatory responses are shaped and unravel immune-suppressive mechanisms which will have broad implications for infectious diseases, autoimmune disorders, metabolic syndromes as well as cancer.
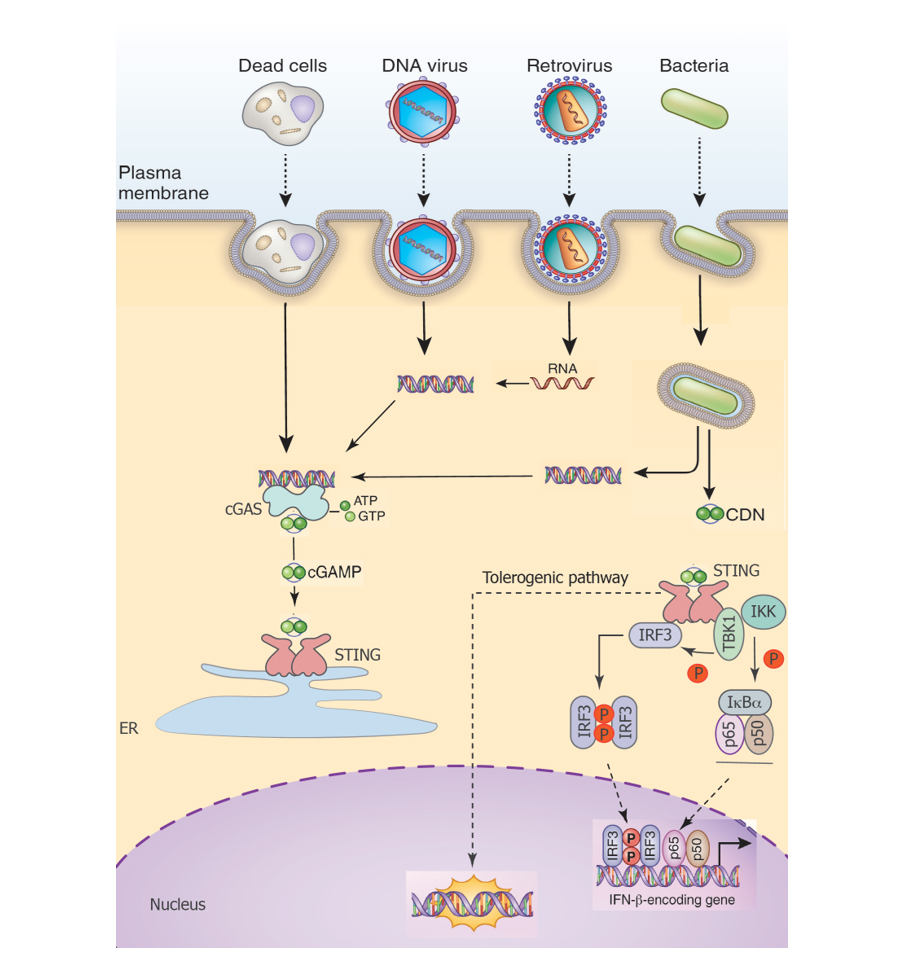
Figure 1. A simple model of how DNA sensing may induce a novel tolerogenic pathway in addition to canonical proinflammatory signaling. It is likely that this pathway is homeostatically engaged and also engaged, perhaps in a temporal manner, during immune responses to pathogens.