The Rachel Buchsbaum Lab
Rac and Tiam 1 Signaling in Cancer
The Rac GTPase has a role in many cellular functions that are deranged in cancer cells, including cell motility and adhesion, cell growth and proliferation, and cell survival and apoptosis. Rac activation triggers diverse signaling pathways, including those governing movements of the actin cytoskeleton, activation of transcription factors, and regulation of the NADPH oxidase complex. GTP-bound Rac mediates these multiple functions through interactions with a host of downstream effector proteins.
Activation of the Rac GTPase occurs through exchange of bound GDP for GTP, catalyzed by one of a number of Rac-specific guanine nucleotide exchange factors (Rac-GEFs). Rac-GEFs all contain similar catalytic DH (Dbl homology) domains adjacent to PH (pleckstrin homology) domains, but differ in their tissue distribution and activation by distinct upstream signals. Tiam1 is a widely expressed Rac-GEF which, like Rac itself, has a role in multiple cellular processes in both normal and malignant cells. Tiam1 promotes invasion in lymphocytes and fibroblasts and adhesion in epithelial cells, and regulates apoptosis in human leukemia cells and axon formation at neuronal growth cones. Tiam1 mediates the effects of Ras transformation on Rac, and is also implicated in signaling pathways involving Src, β-catenin and LEF1/TCF, E-cadherin, and TIMPs (tissues inhibitors of matrix metalloproteinases). To date, all of the myriad effects of Tiam1 are dependent on its ability to activate Rac.
As both Tiam1 and Rac are implicated in multiple downstream effects, mechanisms must exist which determine Tiam1/Rac signaling specificity. Given the explosion of knowledge of signaling pathways over the last two decades, determining mechanisms of signaling specificity and regulation of pathway networks is a major research focus in the field of signal transduction. The regulation of Tiam1 activity is complex, involving multiple phosphorylations, phosphoinositide binding, and modulation of cellular protein levels. However, factors governing the specificity of Tiam1/Rac signaling in terms of downstream events may be more important in determining the ultimate outcome of Tiam1 activation of Rac and the consequences for cellular behavior.
Tiam 1 Influences Rac Signaling Specificity
We have found that Tiam1 itself plays a key role in determining Rac signaling specificity. There have been earlier suggestions that Rac-GEFs play a role in determining signaling pathways downstream of Rac, since expression of specific Rac-GEFs leads to different cellular responses despite similar levels of Rac activation. One mechanism for this is that individual exchange factors can participate in the selection of specific Rac effector proteins for activation. Through participation in protein complexes, Tiam1 determines which downstream pathways are triggered by the Rac molecules it activates. Tiam1 contains N-terminal domains, including adjoining PH and coiled-coil (CC) domains, which participate in a variety of interactions with other molecules. We have found that through these domains Tiam1 binds to at least 3 different families of scaffold protein complexes, leading to distinct downstream signals. Scaffold proteins are large, multi-domain proteins that serve to organize the components of particular signaling pathways in space and time in order to facilitate directed signal transmission. In this case, the scaffold proteins bring an upstream activator of Rac (Tiam1) together with components of signaling pathways downstream of Rac. The presence of Tiam1 at a scaffold complex generates activated Rac in the context of a specific downstream effector, thereby leading to directed signaling downstream of Rac. We have shown that Tiam1 interacts with members of the IB/JIP map kinase scaffold family, leading to activation of p38 and Jnk. The p38 and Jnk map kinases have significant roles in cell survival and apoptosis.
Tiam 1 Regulation
Thus we have identified mechanisms for specifying Tiam1/Rac signaling to pathways affecting cell survival, cell growth, and cell motility, all processes affected in cancer. This leads to two major questions for further investigation. The first is how the various interactions of Tiam1 are regulated. Defining the upstream signals and potential modifications of Tiam1 that lead to its participation in a specific scaffold complex will greatly expand our understanding of what triggers specific Rac functions in cells. Recently we have been using FRET (Fluorescence Resonance Energy Transfer) techniques to study these questions, which have furthered our understanding of the upstream signals regulating Tiam1-scaffold interactions.
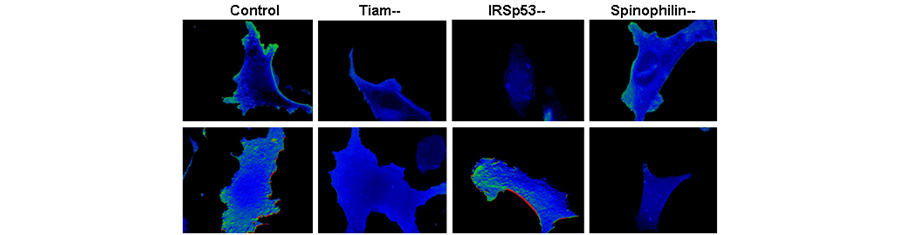
Figure 1. Localization of activated Rac after stimulation detected by fluorescence resonance energy transfer. Green signals indicate areas of Rac activation. After pervanadate or PDGF stimulation (top row), Rac is not activated in cells lacking Tiam1 or IRSp53, while Rac activation is normal in cells lacking spinophilin. After forskolin or epinephrine stimulation (bottom row), Rac activation is preserved in cells lacking IRSp53, but not spinophilin.
Effects on Cell Behavior
The second question is how Rac signaling specificity directed by Tiam1 affects overall cellular behavior. We are using three-dimensional tissue culture models, organotypic culture models, and a mouse model of human breast cancer to study the role of the Tiam1-scaffold protein signaling network in cancer invasion and metastasis. This has led us to develop a novel model of the role of Tiam1 and Rac signaling in cancer cell invasion. We anticipate that precise dissection of how each part of the network contributes to the aberrant behavior of cancer cells will lead to new therapeutic options for treating human cancers.
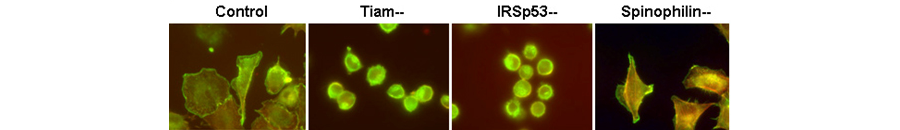
Figure 2. Cortical actin changes and ruffling in cells after replating. Beta (green) and gamma (red) isoforms of actin were visualized by fluorescent tags in cells after replating. In cells lacking Tiam1 or IRSp53, replating is significantly delayed, correlating with Rac activation by pervanadate and PDGF, and indicating increased potential for cell movement.
Tiam1 also interacts with a second scaffold protein, spinophilin/neurabin II, leading to activation of the p70 S6 kinase. S6 kinase plays a key role in cell growth through regulating initiation of protein translation. We have also identified a third interaction involving Tiam1 and IRSp53, an adaptor protein binding to WAVE2. WAVE2 is a scaffold protein for the Arp2/3 complex and mediates actin cytoskeleton, with implications for cell migration and invasion. The same relatively small region of Tiam1 mediates all these scaffold complex interactions. Tiam1 also binds to the hyaluronic acid receptor CD44 and the polarity complex protein Par-3 through this region. Presumably distinct upstream signals trigger differential interactions of Tiam1 with the various scaffold protein complexes, with distinct consequences for downstream signaling. Tiam1 may therefore serve as a key integrating molecule for signals both upstream and downstream of Rac.