The Pamela Yelick Lab
Fishing for Mineralized Craniofacial, Skeletal and Tooth Mutants
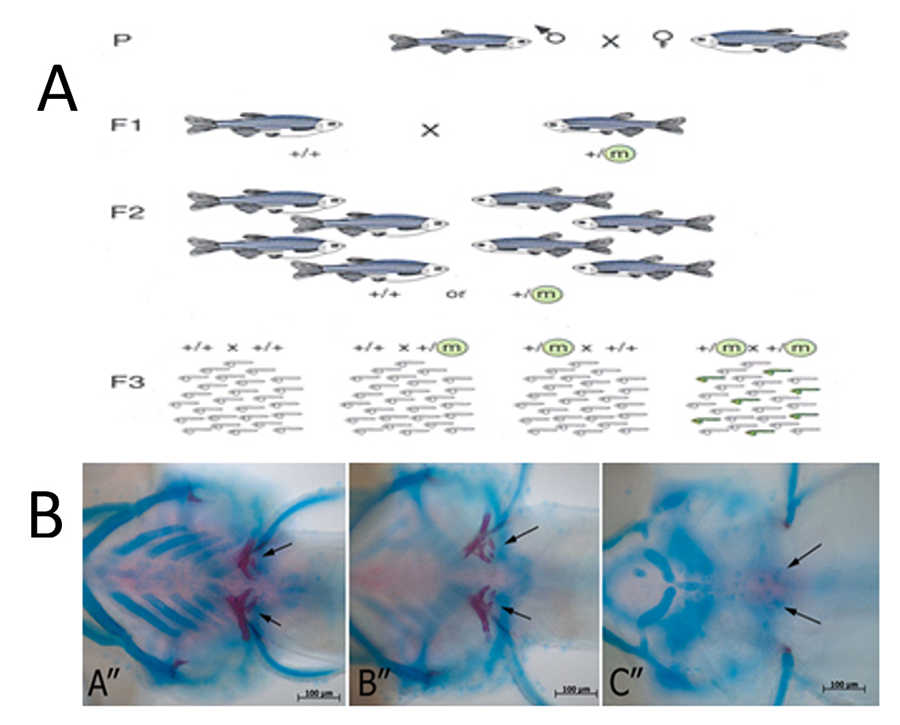
Zebrafish, Danio rerio, are a valuable model for human development and disease due to their significant nucleotide and amino acid sequence identity, and highly conserved gene function. Additional strengths of the zebrafish model include large clutch size, extra-utero fertilization allowing for visual inspection at all developmental stages, optical transparency during early development, rapid growth and relatively inexpensive husbandry. Zebrafish are extremely well adapted for a variety of molecular and genetic techniques including transgenesis and genome editing via CRISPR/Cas9, facilitating analyses that are relatively more difficult to perform in mammals. In addition, the ability to conduct forward genetic saturation mutagenesis screens in zebrafish makes them extremely useful for identifying novel genes required for developmental processes, including mineralized craniofacial, skeletal and tooth development. The fact that zebrafish continuously regenerate teeth throughout their lives also contributes to their utility in elucidating mechanisms for human replacement tooth formation. We are currently characterizing a number of skeletal/craniofacial/tooth mutants identified in a forward genetic mutagenesis screen conducted in the Yelick lab. We anticipate identifying novel genes required for craniofacial/skeletal/tooth development, whose functional characterization will eventually facilitate the design and implementation of clinically relevant therapies to treat a variety of human craniofacial and skeletal dysplasias, including edentulism.
Figure 1. Zebrafish Mineralized Tissue Mutants. (A) Forward genetic chemical mutagenesis approach to identify genes regulating mineralized craniofacial/skeletal/tooth development and regeneration. (B) Craniofacial and tooth defects identified via Alcian blue and Alizarin Red staining.
Roles for acvr1l in Mineralized Tissue Formation
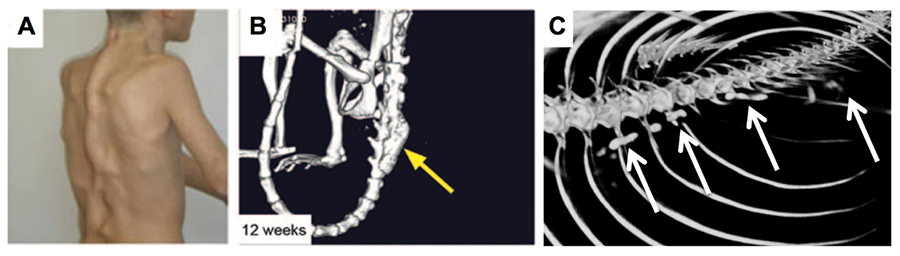
To elucidate roles for TGFβ signaling in craniofacial/skeletal/tooth development, early efforts in my laboratory focused isolating and characterizing zebrafish type I and II TGFβ family member receptors. These studies identified a variety of type I and II receptors, including alk8/acvr1l. Functional studies of wild type, constitutively active and dominant negative alk8/acvr1l isoforms revealed roles in neural crest cell formation and mediolateral specification, early dorsoventral patterning of the embryo, and in later developmental events including zebrafish primary and replacement tooth development, and craniofacial and skeletal cartilage and bone formation. Zebrafish laf/alk8/acvr1l mutants also exhibit defects in replacement tooth formation. We have recently begun characterizations of zebrafish models for human Fibrodysplasia Ossificans Progressiva (FOP), a debilitating and lethal disease characterized by explosive heterotopic ossification caused by activating mutations in human ACVR1, the human ortholog of zebrafish acvr1l. We anticipate that zebrafish FOP models will provide invaluable insight into the etiology and signaling pathways contributing to FOP, and eventually provide new therapeutic treatments for HO in a variety of human conditions.
Figure 2. Fibrodysplasia Ossificans Progressiva (FOP). (A) Human, (B) mouse and (C) zebrafish models for FOP.
Characterizing the Zebrafish Dental Stem Cell Niche
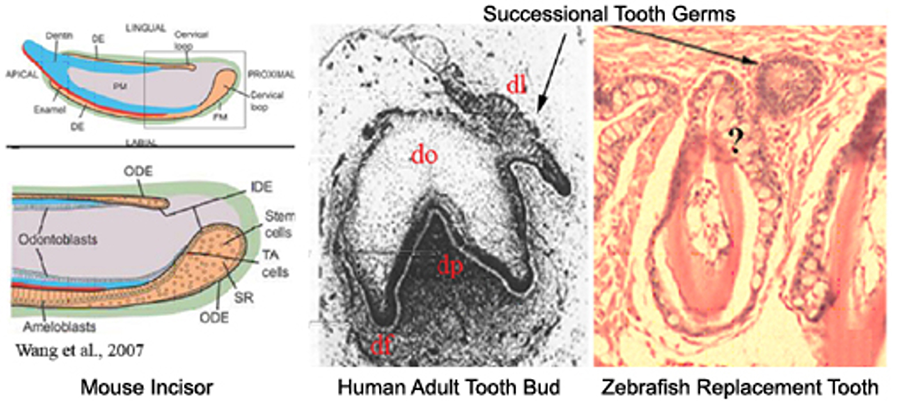
We are also working to identify signaling pathways regulating dental stem cell maintenance and differentiation in zebrafish primary and replacement tooth development, for comparison to ongoing studies in mouse and humans.
Figure 3. Do similar genes regulate primary and replacement tooth in mice, humans, and zebrafish? Mouse incisor: The continuously erupting incisor model has been used to identify genes regulating dental epithelial progenitor cell differentiation. Successional human tooth formation and zebrafish replacement tooth formation. Abbreviations: DE, dental epithelium; df, dental follicle; dl, dental lamina; do, dental organ; dp, dental papilla; IDE, inner dental epithelium; ODE, outer dental epithelium; ST, stellate reticulum; TA, transit amplifying cells.
Mammalian Tooth Tissue Engineering
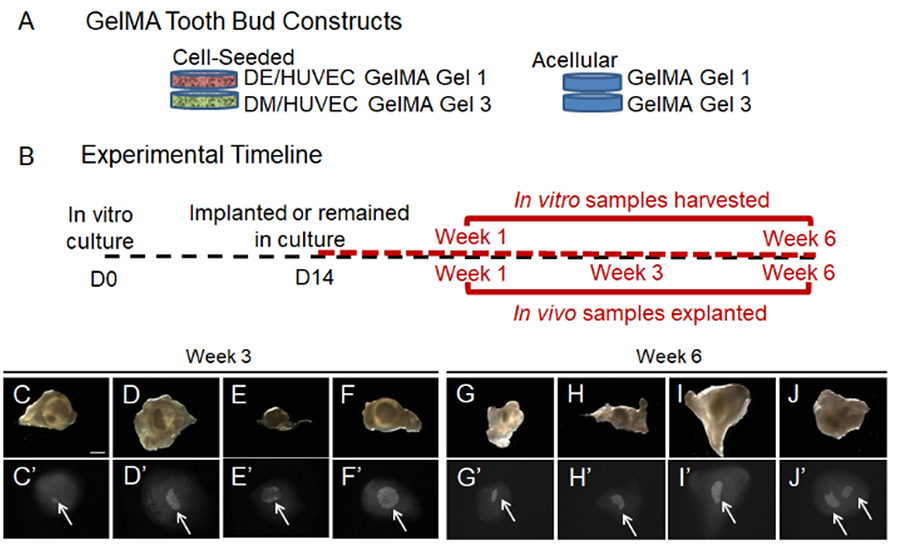
In a long standing collaboration with Dr Joseph Vacanti, MD, Chief of Surgery at Massachusetts General Hospital, Harvard Medical School, we are investigating approaches to bioengineer biological tooth substitutes using state-of-the-art tissue engineering techniques. We use primary human dental pulp cell cultures, seeded onto biodegradable hydrogel and natural tooth bud ECM scaffolds and implanted in animal hosts, to form bioengineered teeth. To date, we have succeeded in generating organized bioengineered teeth composed of organized dental pulp, odontoblasts, dentin, ameloblasts, enamel, cementum and immature tooth roots. We are currently working to scale up current models to generate full-sized human tooth structures of specified size and shape, using a variety of scaffold materials and designs. In addition to whole tooth tissue engineering, we anticipate that these studies will result in a variety of new therapies including bioengineered reparative dentin and enamel, and dental pulp.
Figure 4. Three dimensional Bioengineered Tooth Models. (A) GelMA tooth bud constructs. (B) In vitro culture and in vivo implantation. (C-J) Mineralized tissue formation. For more information, see Smith et al, 2016.