The M. Elizabeth Fini Lab
Eye Biology and Disease
The eye is a remarkable organ. Its importance to quality-of-life cannot be overstated. As much as 80% of what we learn is through our eyes. As much as 80% of our memories are determined by what we see.
Eyes made their appearance about 550 million years ago as patches of photosensitive protein in single-celled organisms. Over the millennia, the spots evolved into something more. In modern humans, the eye is the second most complex organ after the brain, to which it is directly connected. The eye can be likened to a “smart” digital camera, processing images it receives before projecting them upon the neural network of the brain’s visual center.
As a model organ, the eye has something for every biomedical scientist. As examples, developmental geneticists discovered in the eye the first ‘‘master control gene’’, a long-sought developmental regulator with the capacity to switch on an entire genetic program for organ formation. Special features of the cornea have made it an important model for cell biological breakthroughs in regeneration and stem cell biology. Neuroscientists investigate the retina and as the most accessible part of the central nervous system. The aqueous outflow pathways, a marvel of hydrodynamics, still hold many mysteries currently under intense investigation by molecular biologists, biophysicists and bioengineers. Each of these areas has been of interest to the Fini lab over the years (e.g., master control gene Pax6: Fini et al, 1997; Sivak et al, 2000; Sivak et al, 2004; Bassett et al, 2007; corneal regeneration: Matsubara et al, 1991; West-Mays et al, 1995; Stramer et al, 2003; stem cells: Tsai et al, 2011; Wu et al, 2015; stroke to the eye: Chintala et al, 2002; Santos et al, 2012; ocular hypertension: Wang et al, 2001 and News & Views highlight, March 2001, Nature Medicine.
Figure 1. Nature Medicine, Volume 7 Issue 3, March 2001 (cover). Little is known about the molecular mechanisms responsible for the development of glaucoma, the leading cause of irreversible blindness. On page 304 of this issue, Wang et al. report that expression of the cell-adhesion molecule endothelial leukocyte adhesion molecule-1 (ELAM-1) is upregulated in cells from the outflow chamber of glaucomatous eyes, making it the first molecular marker of human glaucoma. The image shows the anterior chamber of a glaucoma patient's eye, viewed through a mirrored contact lens. The darkly pigmented tissue is a typical symptom of pigmentary glaucoma.
Like all organs of the body, eyes are subject to dysfunction. The 2016 National Health Interview Survey (NHIS) data release documents an estimated 25.5 million adult Americans equal to about 10% of all adult Americans – reported they either "have trouble" seeing, even when wearing glasses or contact lenses, or that they are blind or unable to see at all.
My lab trains young scientists in current molecular and cell biological approaches to the study of eye biology and disease. We use cell culture and mouse models, and also analyze human tissues and DNA. Since disease mechanisms share many commonalities across organ systems, our findings often lead us to study disease processes outside of the eye, as well (e.g. Kupferman, 2000; Wang et al, 2000; Asahi et al, 2001; Johnson et al, 2004; Mukherjee et al, 2006. I am increasingly committed to drug discovery to provide for better treatments.
Current projects are described below.
The Ocular Surface
The ocular surface is a complex and delicate arrangement of mucosal organs at the front of the eye that provides the moist environment necessary for corneal clarity and visual acuity. Diseases of the ocular surface are among the top reasons for visits to eye care practitioners. These conditions disrupt the normal progression, maturation and turnover of the ocular surface epithelia and can severely affect eyesight and quality of life. Ocular surface damage is assessed by staining with vital dyes (fluorescein, rose bengal, or lissamine green), considered a “sign” of disease. Disease symptoms may include blurry vision, discomfort or pain, redness and itching, and in severe cases, blindness due to corneal scarring.
Protection of the Ocular Surface in Dry Eye
Many ocular surface diseases are initiated by loss of tear film homeostasis and can be grouped under a syndrome known as dry eye. Tear dysfunction leads to desiccating stress at the ocular surface, initiating an autoimmune-like “vicious cycle of inflammation” that damages the ocular surface barrier. The cycle is recognized as the core driver of the pathologic process.
Anti-inflammatory steroids and small molecules targeting autoimmune T-cell function are currently the only FDA-approved therapeutics for dry eye. These drugs are not effective in all patients and each has major drawbacks.
Dry eye is a global problem, afflicting over 30 million people in the United States alone, and at least 344 million people worldwide. Prevalence is higher among women than men and increases with age, but is also notable among the 18-34-year age group. It has been estimated that the overall burden of DE for the US healthcare system is $3.84 billion. These facts underpin the great need for new treatments.
Current opinion holds that breaking the inflammatory vicious cycle at alternative entry points is the key to new treatments for dry eye. Direct protection of the ocular surface barrier could provide such a new entry point. In addition, targeting of the acute effects of desiccating stress by directly protecting the ocular surface would provide rapid relief of eye irritation in acute situations where chronic dry eye is exacerbated by environmental, and other acute factors.
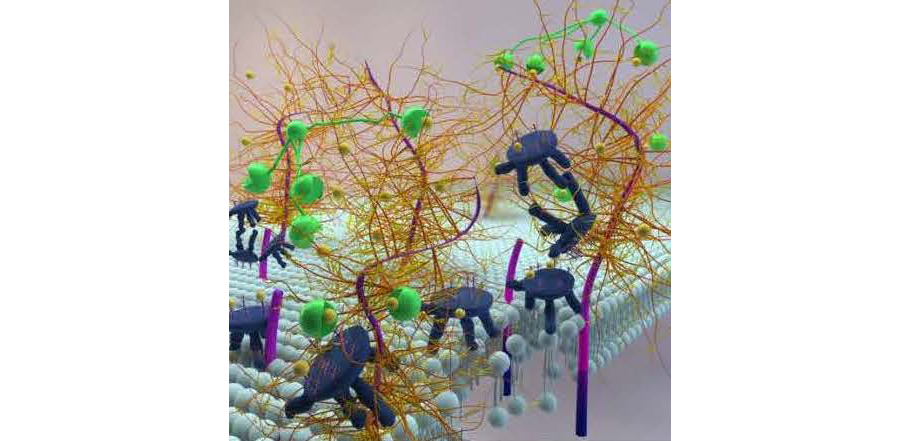
We are currently investigating protection of the ocular surface in dry eye. We are focused on two different molecules. The first is the natural tear protein clusterin, a secreted molecular chaperone and inhibitor of MMP9, a key mediator of ocular surface disease in dry eye (Pflugfelder et al, 2005; Jeong et al, 2012; Bauskar et al, 2015; Fini et al, 2016; Yu et al, 2018). Clusterin binds to the ocular surface and protects, seals and heals (Fig 1). The second is molecule is Dynasore, a small molecule that targets dynamin family proteins. Both are candidates for use in the treatment of dry eye (Webster et al, 2018).
Figure 1. Conceptual model depicting CLU binding to areas of barrier disruption at the ocular surface subjected to desiccating stress. Membrane-Associated Mucins (fuscia, dark blue and gold), LGALS3 (green) and CLU (dark blue with blue and coral “antlers”) are shown interacting with one another, and with the lipid bilayer of the apical epithelial cells (light blue), in this artist's conception of the ocular surface. Membrane-Associated Mucins are depicted as long, flexible rods (fuscia) traversing the lipid bilayer of the apical epithelial cells of the ocular surface, with their intracellular domains projecting into the cytoplasm (blue). The carbohydrate chains (gold) linked to the extracellular domains are extensively branched. Following exposure to desiccating stress, membrane-associated mucins may be proteolytically cleaved, leaving membrane-embedded protein “stubs” (fuscia). LGALS3 molecules (green) are shown with the C-terminal carbohydrate-binding domain appearing as a “mouth” linked to the N-terminal multimerization domain by a long thread. Some of these LGALS3 molecules are depicted as self-associating via their multimerization domains, a requirement for network formation and exclusion of clinical dyes. In other cases, the multimerization domain is drawn as proteolytically cleaved, leaving only the carbohydrate-binding domain. CLU molecules (blue) are schematically modeled after a milking stool. The “seat” of the stool represents the disulfide-bonded region of the polypeptide chains decorated by carbohydrate chains (blue and coral) emanating from six attachment sites. The three legs of the stool represent the C-terminal and N-terminal portions of the molecule containing the amphipathic helices. The “arm” of the stool is the C-terminal portion lacking an amphipathic helix. Galactose moieties on both the mucin and CLU carbohydrate chains are depicted as small “marbles” (yellow). The carbohydrate-binding domains (“mouths”) of LGALS3 molecules are shown binding to (“eating”) the yellow globes. CLU molecules are shown in various interactions 1) self-associating, 2) binding to the lipid bilayer, and 3) associating with proteolyzed mucin “stubs”. In the foreground, the proteolytically cleaved carbohydrate-binding domain of an LGALS3 molecule is shown binding to a marble on a carbohydrate chain of a CLU molecule. This drawing aims to illustrate the idea that all-or-none sealing of the ocular surface barrier disrupted by desiccating stress occurs when the concentration of CLU molecules is high enough to compete effectively with mucins for binding to LGALS3 molecules. Adapted from the doctoral thesis of Aditi Bauskar, University of Southern California. Image created by graphic artist Ella Maruschenko.
Novel Mucins at the Ocular Surface
Mucosal tissues throughout the body are defined by mucins – large, heavily O-glycosylated proteins present at the interface between wet epithelia and the external environment, including the respiratory, oral, gastrointestinal and vaginal tracts, as well as the ocular surface. The mucosal glycocalyx forms a barrier to the outside world, defending and protecting, while also allowing selective penetration. A comprehensive structure/function characterization is extremely important if we are to manipulate the glycocalyx to therapeutic advantage, e.g., for drug delivery or to thwart pathogen penetration strategies.
My lab is currently generating structure/function information about two newly-discovered membrane-associated mucins (MAMs) expressed at the ocular surface and linked to ocular surface disease: MUC21 and MUC22. MAMs have classic cell surface receptor-like structures and extracellular domain (ECD) cleavage transduces a signal to the intracellular domain (ICD) to activate signaling pathways that regulate inflammation, cell-cell interactions, differentiation, and apoptosis. There is substantial genetic and expression evidence for involvement of MUC21 and MUC22 in mucosal diseases of the ocular surface. These data and inferences from DNA sequence analysis suggest an immunomodulatory role in dampening inflammation and in control of secreted mucous accumulation.
We propose that MUC21 and MUC22 respond to specific pathogens or other danger signals by shedding their ECDs, thus initiating interaction with toll-like or related receptors. This results in specific phosphorylation of the ICD, launching signal transduction cascades to fulfill their roles. Our approach to address this hypothesis employs proteomics technology, which has never been used to study the ICD of MAMs, incorporated into an experimental strategy designed to enhance specificity and focus.
Ocular Hypertension/Glaucoma
Ocular hypertension is the major risk factor for glaucoma, the third most prevalent cause of visual impairment and blindness among Caucasian-Americans and the leading cause among African-Americans. While effective therapeutics exist in the form of eye drops or surgical intervention, it is often the case that patients do not reach their intraocular pressure target, indicating a continuing unmet medical need.
Intraocular pressure is a function of aqueous humor dynamics. All forms of high tension glaucoma are specifically due to impaired aqueous outflow via the conventional pathway through the trabecular meshwork and Schlemm’s canal. In open angle forms of disease, the defect appears to be at the cell and tissue level, although exactly what goes wrong is still not well understood. Risk factors include genetic variation, physiologic stress and aging.
Pharmacogenomics of Steroid-Induced Ocular Hypertension
Ocular hypertension is the major risk factor for glaucoma, the third most prevalent cause of visual impairment and blindness among Caucasian-Americans and the leading cause among African-Americans. While effective therapeutics exist in the form of eye drops or surgical intervention, it is often the case that patients do not reach their intraocular pressure target, indicating a continuing unmet medical need.
Sensitivity to glucocorticoids appears to be genetically determined, thus, steroid-induced ocular hypertension can be investigated using genetic/genomic approaches that requires no a prior biological knowledge. Importantly, steroid-induced ocular hypertension has the advantages of a much shorter observation period and much larger effect sizes than primary open angle ocular hypertension. Therefore, the study of steroid-induced ocular hypertension could make it possible to discover common pathways controlling intraocular pressure and contributing to ocular hypertension that would not have been revealed any other way. In addition, sensitivity to steroid-induced ocular hypertension is important to study in its own right, as it limits the usefulness of a very effective class of anti-inflammatory drugs.
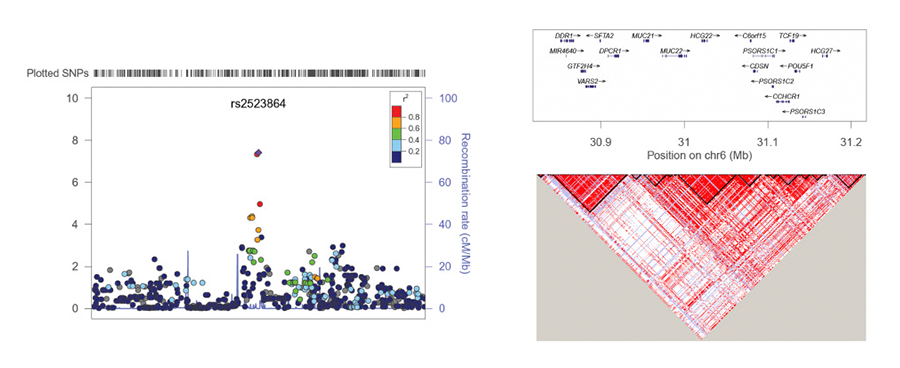
A candidate gene association study from our lab for known glucocorticoid receptor polymorphisms associated with steroid-induced ocular hypertension yielded negative results (Gerzenstein et al, 2008). However, when we applied a more powerful and broad-based pharmacogenomics approach, we identified a significantly associated SNP linked to the novel mucin gene HCG22 (Fig 2).
Figure 2. Regional Association Plot for lead SNP rs2523864 (marked as purple diamond) on Chr6. All SNPs shown as circles are plotted with their respective P values against their genomic location. The solid diamond indicates the top-ranked SNP rs2523864, which maps upstream of the novel mucin gene HCG22. The colored box at the right or left corner of each plot indicates the pairwise correlation (r2) between the top SNP and the other SNPs in the region. The grey circles indicate the imputed SNPs from the CEU population of the HapMap. Each plot was created using LocusZoom (http://locuszoom.sph.umich.edu/) for the top-ranked SNP in each region with a 400-kb region surrounding it. SNPs are plotted at the top of the figure. The box on the right plot shows the gene annotations in the region, with the arrow indicated the DNA strand for transcription. The lower right LD plot was created using Haploview (https://www.broadinstitute.org/haploview/haploview). From: Identification of a Novel Mucin Gene HCG22 Associated With Steroid-Induced Ocular Hypertension. Jeong S, et al. Invest Ophthalmol Vis Sci. 2015 Apr;56 (4):2737–48. doi: 10.1167/iovs.14–14803. Used with permission from the publisher. The authors acknowledge the Association for Research in Vision and Ophthalmology as the copyright holder.
HCG22 is the first gene linked to steroid-induced ocular hypertension(Jeong et al, 2015; Fini et al, 2017). This success, gave us the confidence to initiate a larger study, taking the same approach. For this we have partnered with a large clinical practice that maintains a registry of demographically homogenous patients, all treated with pharmaceutical glucocorticoids in a highly uniform way following surgery, with careful pre-surgical characterization and post-surgical follow-up. Thus, these patients are fully phenotyped, representing a unique and powerful resource. We are adding DNA sequencing to the previously used microarray-based genotyping to enhance discovery opportunity.
GPR158
G protein-coupled receptors (GPCRs) comprise the largest class of cell surface receptors in humans. Activated GPCRs bind heterotrimeric G proteins, resulting in production of second messengers and downstream intracellular signaling. GPCRs are by far the most successful targets for pharmacotherapy to date, representing 30 to 40% of marketed pharmaceuticals. GPCRs are targeted by most glaucoma drugs in current use.
Many novel orphan GPCRs without known ligands were identified by sequencing of the human genome, providing for potential new drug targets. Our laboratory identified GPR158 in our pharmacogenomic screen for genes associated with susceptibility to steroid-induced ocular hypertension, discussed above. Sequence analysis classified the protein as a distant member of GPCR family C, which includes receptors for the neurotransmitters glutamate and gamma-aminobutyric acid (GABA), as well as the androgen and BGLAP (osteocalcin) receptor GPRC6A.
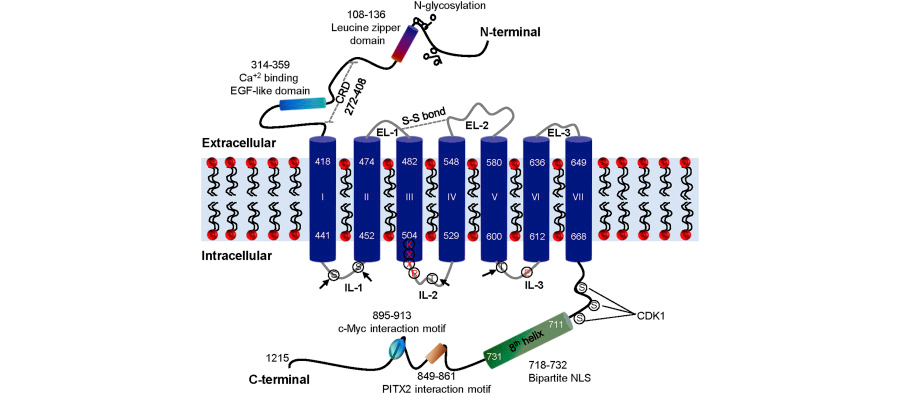
We were able to deduce a substantial amount of information about GPR158 structure from DNA sequence information (Fig 3). Consistent with the proposed role, glucocorticoids stimulate GPR158 expression by trabecular meshwork cells in culture (Patel et al, 2013). In turn, when ectopically over-expressed in these cells, GPR158 itself stimulates cell proliferation. Correspondingly, knockdown of endogenously-expressed GPR158 inhibits cell proliferation. GPR158 over-expression also increases barrier function of a cell monolayer, also consistent with ocular hypertension.
Figure 3. Predicted features of the protein encoded by GPR158. The schematic shown is based on sequence analysis of the conceptually translated product of GPR158. Three extracellular loops (ELs) and three intracellular loops (ILs) connect the seven TM (numbered I-VII). The arrow indicates putative PKC and PKA phosphorylation sites in the ILs. The cysteine residues in EL-1 and EL-2 involved in disulfide (S–S) bond formation are shown as a dotted grey double line. The eighth helix, bipartite NLS, c-Myc and PITX2 interaction motifs, and putative phosphorylation sites for kinases, such as CDK1, are indicated in the C-terminal tail. The leucine zipper domain, EGF like domain, N-glycosylation sites and putative CRD are shown in the N-terminal of GPR158. The conserved amino acid residues, KXXR and E, involved in G protein activation in class C GPCRs are marked in red color. CDK1, cyclin-dependent kinase 1; CRD, cysteine rich domain; EGF, epidermal growth; EL, extracellular loop; IL, intracellular loop; NLS, nuclear localization signal; PITX2, paired-like homeodomain transcription factor 2. From: GPR158, an orphan member of G protein-coupled receptor Family C: glucocorticoid-stimulated expression and novel nuclear role. From: Patel et al, 2013. CC-BY; used with permission from the publisher.
GPR158 is most prominently expressed in the central nervous system. At about the same time we published our first paper on GPR158, another lab also discovered this, as well as the closely related Gpr179, in a screen for proteins that form complexes with the GTPase-activating protein RGS7 in brain and retinal neurons of mice. Significantly, GPR158 was recently linked functionally to cognition and anxiety-like behaviors in mice, as well as chronic stress and depression in mice and humans.
We found GPR158 also affects cell proliferation in prostate cancer cell lines, with expression stimulated by a second steroid hormone, androgen, leading to increased androgen receptor expression (Patel et al, 2015). Alternatively, androgen receptor pathway inhibition, leads to a high level of GPR158 expression over a period of weeks, coincident with induction of neuroendocrine differentiation markers. In addition, high level GPR158 alone induced neuroendocrine differentiation, which is linked to disease progression (Patel et al, 2015). Very recently, GPR158 was also linked to progression of glioma in the brain.
In our latest study, we investigated GPR158 function in the visual system (Itakura et al, 2019). We showed prominent expression specifically in the visual center of the cerebral cortex. Expression was also observed in the eye, including photoreceptors, ganglion cells and trabecular meshwork. Protein was also localized to the outer plexiform layer of the neural retina. Gpr158 deficiency in knockout mice conferred short-term protection against the intraocular pressure increase that occurred with aging, but this was reversed over time. More strikingly, the pressure lowering effect of the acute stress hormone, epinephrine, was negated in knockout mice. In contrast, no disruption of the electroretinogram was observed. Gene overexpression in cell cultures enhanced cAMP production in response to epinephrine, suggesting a mechanism for intraocular pressure regulation. Overexpression also increased survival of cells subjected to oxidative stress linked to ocular hypertension, associated with TP53 pathway activation. These findings implicate GPR158 as a homeostatic regulator of intraocular pressure and suggest GPR158 could be a pharmacological target for managing ocular hypertension.