The Lidija Covic Lab
Protease-Activated Receptors in Cancer and Vascular Cell Biology
In the Covic laboratory, we study a novel class of cell surface proteins known as protease-activated receptors. These receptors include the major thrombin receptor of blood vessels and platelets as well as receptors that are involved in cancer invasion and metastasis. The first member of the protease-activated receptor family, PAR1, has been identified as an oncogene and its expression correlates strongly with invasive and metastatic processes of cancer cells from breast, melanoma and ovarian tumors. In addition, we study the downstream signaling molecule, the angiogenesis factor Cyr61, in the malignant progression of breast cancer. Cyr61 was identified as a marker for poor prognosis in a recent large breast cancer study.
Matrix Metalloproteases and Cyr61 in Tumorigenesis
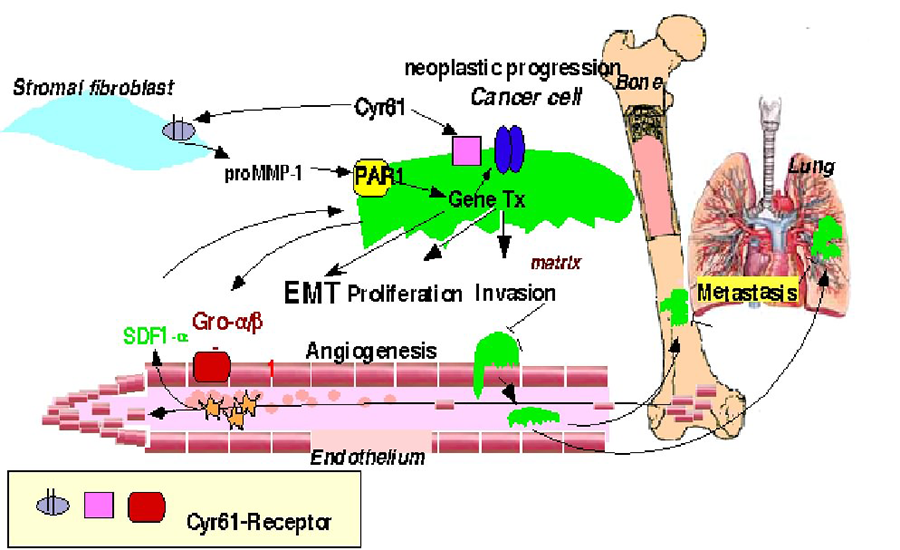
Our laboratory studies regulation of matrix metalloproteases (MMPs) and their expression by tumor derived angiogenic factor Cyr61. We recently determined that stromal-derived MMP-1 also acts as a signaling molecule by cleaving protease-activated receptor 1 (PAR1) to cause breast cancer cell migration and invasion. We show that ectopic PAR1 expression induces expression of the angiogenic factor Cyr61(CCN1) in breast cancer cells. The tumor-derived Cyr61 acts as an invasogenic signaling molecule that induces MMP-1 expression in adjacent stromal fibroblasts. We hypothesized that tumor-derived Cyr61 may have a global paracrine function in regulating pro-invasogenic lignds (MMPs, chemokines, growth factors) production from adjacent stromal cells which may contribute to tumor invasion through multiple signaling pathways including PAR-1 as schematically shown below.
Figure 1. A schematic of the signaling pathways affected by Cyr61 during tumorigenesis.
The Role of PAR1 in Metastasis
Our laboratory also studies breast cancer transformation by PAR-1 with a particular focus on the invasive and metastatic singling mechanisms. PAR1, a G protein coupled receptor, had been previously identified to be a highly potent transforming gene in 2001 (for a review see Whitehead et al, 2001) but the mechanism of action of its oncogenic activity was largely unknown. We study ectopic expression of PAR1 in human breast cancer cells induced epithelial-mesenchymal-transition (EMT) and its activation in global gene transcription. We are using PAR-1 ectopic expression system to determine critical signaling for development of invasive and metastatic breast cancer phenotype. Using in vivo mouse xenograft models, our major goal is to identified new therapeutic targets to treat metastatic breast cancer.
CXCR4 Antagonism in Stem Cell Mobilization, Chemosensitization and Metastasis
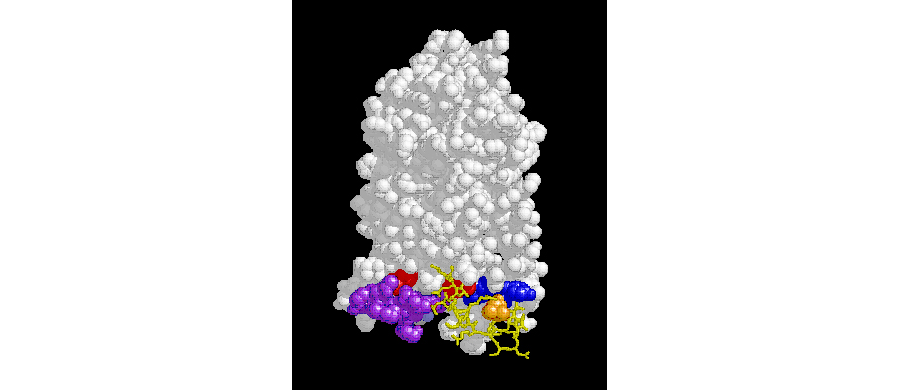
A subset of cancer cells might have unique quality that may propel metastasis of tumors. Stromal cell-derived factor-1 (SDF-1 also know as CXCL12) and its receptor CXCR4 play a central role in both of these processes. Disruption of the SDF1a/CXCR4 interaction releases hematopoetic stem cells from the bone marrow into peripheral blood. Here, we use pepducin technology to target intracellular loops of CXCR4 (see Figure 2) in order to develop new generation of inhibitors for studies in cancer.
Figure 2. The structural model of the 7-transmembrane CXCR4 receptor illustrating the targeted intracellular loops.