The Lauren Black Lab
Engineering Approaches to Cardiovascular Disease
Research in the Black Lab focuses on three major aspects of investigation related to cardiovascular biology and disease.
The Extracellular Environment in Stem Cell Differentiation and Cardiac Function
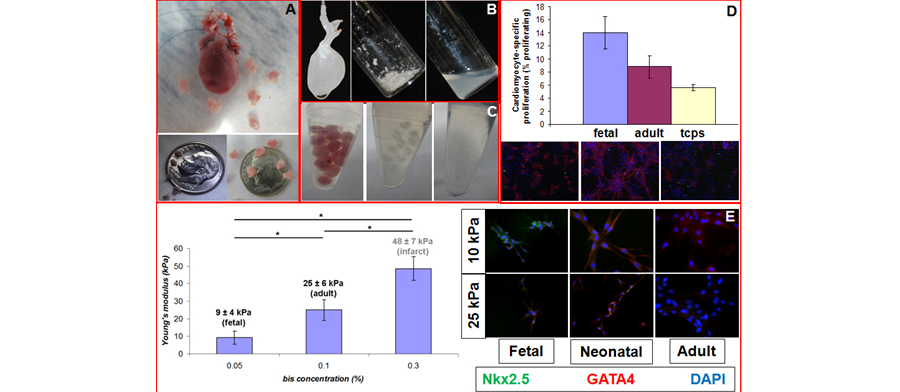
Our current work in this area centers on investigating the role of the cardiac extracellular matrix (ECM) in the differentiation and maturation of stem cells to cardiomyocytes, with a potential end goal of developing an in vitro platform for therapeutic applications. We are currently exploring the mechanotransductive and integrin-mediated signaling effects of different developmental aged ECM composition and stiffness on the genetic regulation of cardiac differentiation. Additional projects include investigating intra-cellular signaling mediated by specific integrin binding domains, and re-cellularizing perfusion decellularized hearts.
Another focus area in this part of the lab's work is the use of this platform for tissue engineering and regenerative medicine approaches to treat children suffering from congenital heart defects. A major challenge to the development of engineered cardiac tissue is the low regenerative potential of mature cardiomyocytes - cardiomyocytes are highly proliferative in the fetus, but stop proliferating soon after birth. Preliminary data demonstrate that plating neonatal rat cardiomyocytes on fetal cardiac ECM that has been adsorbed onto tissue culture plates results in enhanced maintenance of proliferation of cardiomyocytes as compared to adult cardiac ECM or tissue culture polystyrene (TCPS). The long-term goal of this part of the project is to develop new heart tissue for children either in the lab or to use ECM as a biomaterial to promote heart regeneration in vivo. Future work in this area will seek to study the effects of mechanical and electrical stimulation on cardiac differentiation.
Figure 1. (A) Isolated adult and fetal E19 hearts (top). Fetal E15 (bottom left) and E19 (bottom right) hearts. Decellularization and solubilization of adult (B) and fetal (C) heart ECM. (D) Autofluorescence of adult ECM absorded of TCPS (left). Neonatal rat cardiomyocytes (nnrCMs) on fetal ECM stained with Hoechst (blue), α-actin (red) and PHH3 (green). Inset shows proliferating CM (overlay of Hoechst and PHH3 appears yellow). NnrCMs on RCPS control (right). Inset shows representative cell. (E) (left) Alterations in polyacryamide (PAam) gel stiffness as a function of bis-acrylamide concentration. Note that we can mimic developmental aged tissue stiffness as well as pathological stiffness. (right) Mesenchymal Stem Cells (MSCs) plated on PAam gels of different stiffnesses (vertical) and composition (horizontal) stained for cardiac transcription factors GATA 4 and Nkx2.5. Note that low stiffness and early developmental time point ECM results in robust co-expression of these two markers - a response typical of normal cardiac development.
New Methods for the Creation and Culture of Engineered Myocardial Tissues
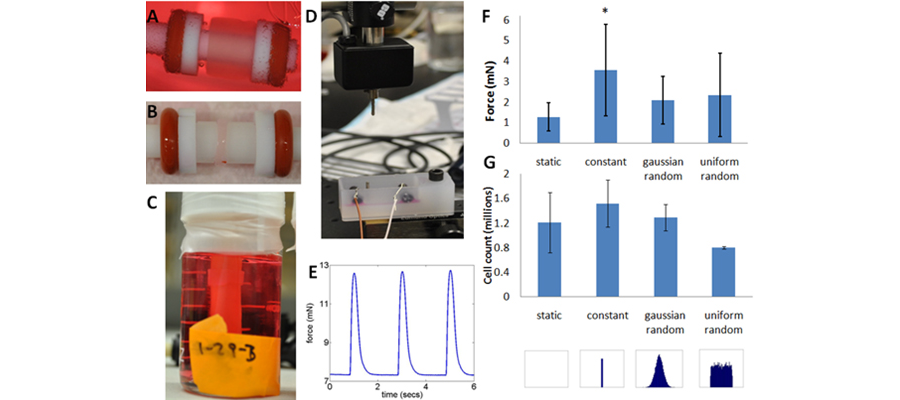
We are interested in understanding how combined mechanical and electrical stimulation affect engineered myocardial tissue used for improving cardiac function after a myocardial infarction. One important aspect of native tissues that has not been explored is the effect of variability and how it influences tissue function. Currently, our studies involve determining if and how variability in mechanical stimulation regimens affects the development of the tissue. To do this, we have created a custom distention bioreactor system that allows for high levels of control over the frequency and strain of the mechanical stimulation. Ultimately, we will add electrical stimulation to the bioreactor system and use combined mechanical and electrical stimulation to probe the mechanisms of physical stimulation on myocardial tissue.
Figure 2. System for Stimulating Engineered Myocardial Constructs. (A) Fibrin gel based engineered myocardial construct on mandrel immediately after casting and after 2 weeks in culture (B). (C) A construct in the bioreactor after 10 days in culture under mechanical stimulation. Note that the tube in the middle of the jar is distensible latex that is inflated with air pressure to stretch the construct. (D) An image of the contraction force measurement system and an example time course of contraction force of an engineered myocardial construct from the constrant stimulation group (E). (F) Contractile forces of constructs after two weeks of mechanical stimulation under different frequency stimulation regimes and corresponding cell counts of constructs from these groups via a DNA assay. Note that while the constant force is significantly higher in the constant mechanical stimulation group as compared to the static constricts, there is no significant difference in final cell number indicating that the increased force is due to cell hypertrophy caused by mechanical stretch.
Cardiac Disease Models that Incorporate Alterations to the Extracellular and Biophysical Environment that Accompany Disease
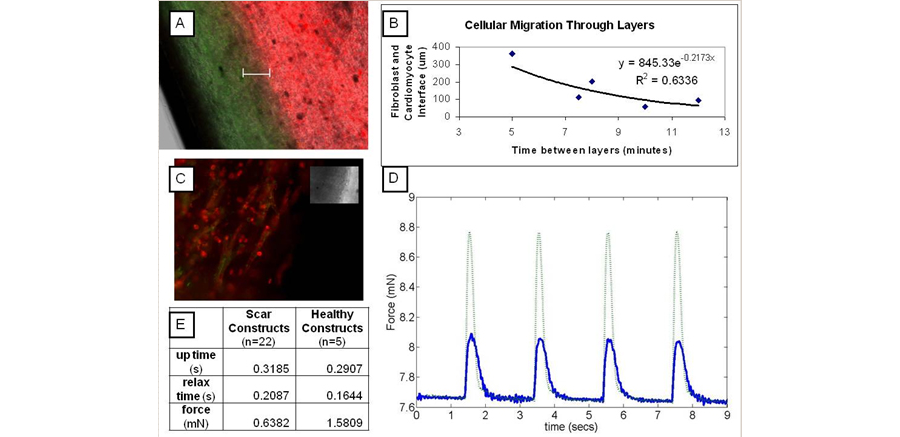
One of the innovative methods for treating myocardial infarction and heart failure is cell therapy, which aims to repair the infarcted tissue by injecting stem cells into the infarct in order to regenerate cardiac muscle. However, the effectiveness of this approach is limited because only 5% of injected cells are retained, viability is often low, and their differentiation to cardiac muscle cells is incomplete. Therefore, new methods for cellular attachment must be elucidated. Another area of research focus in the lab is on the development of an in vitro model of the negative remodeling process following MI and the post remodeling environment during progression to heart failure. Such models would improve researchers’ ability to prevent the progression to heart failure by allowing for the evaluation of new methods to enhance cell delivery, survival and differentiation in a high throughput manner. Currently we have adapted the fibrin gel engineered myocardial system above to generate layered constructs that mimic the stiffer, fibroblast filled infarct in between two areas of healthy engineered myocardium. Future research seeks to study the electrical properties of this in vitro disease model as well as assessing the impact of this environment on stem cells differentiation and maturation to cardiomyocytes.
Figure 3. A Three Dimensional Myocardial System that Has the Potential to Mimic the Heterogenouse Nature of Infarcted Heart Tissue. (a) Histology of an engineered myocardial construct with 8 minute time points between healthy and scar layers. Cells labeled with membrane dyes (fibroblasts, green, cardiomyocytes, red). White bar (200 μm) indicates layer interface. (B) The negative exponential relationship between the size of the interface and time between the addition of each layer of construct. (C) Construct (7 min layers) fluorescently labeled red for Myosin Heavy Chain and green for Connexin-43 (inset if transmitted). (D) Representative contraction forces (solid blue is scar and dotted green is healthy). (E) Contraction forces of the model infarct and healthy constructs.