The Larry Feig Lab
Ras Pathway Proteins
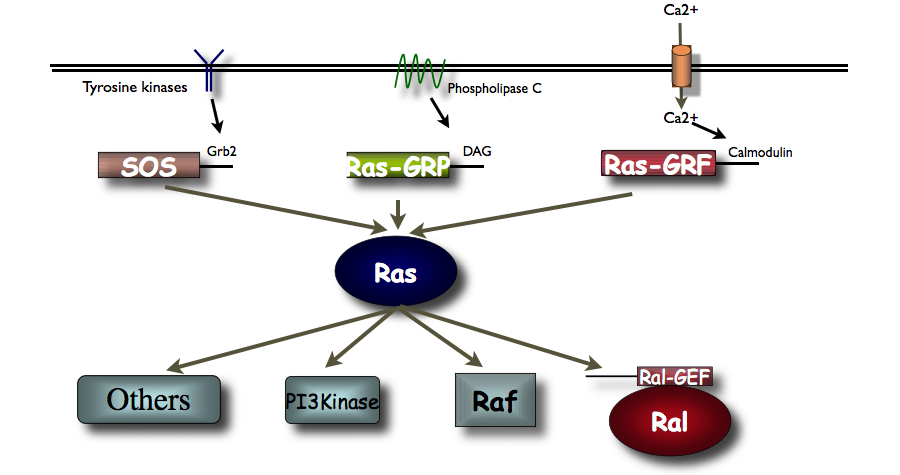
Figure 1. The diagram presents an overview of signaling via Ras pathway proteins.
Ras-GRF1 Controls the Stress Response Specifically in Adolescent Females
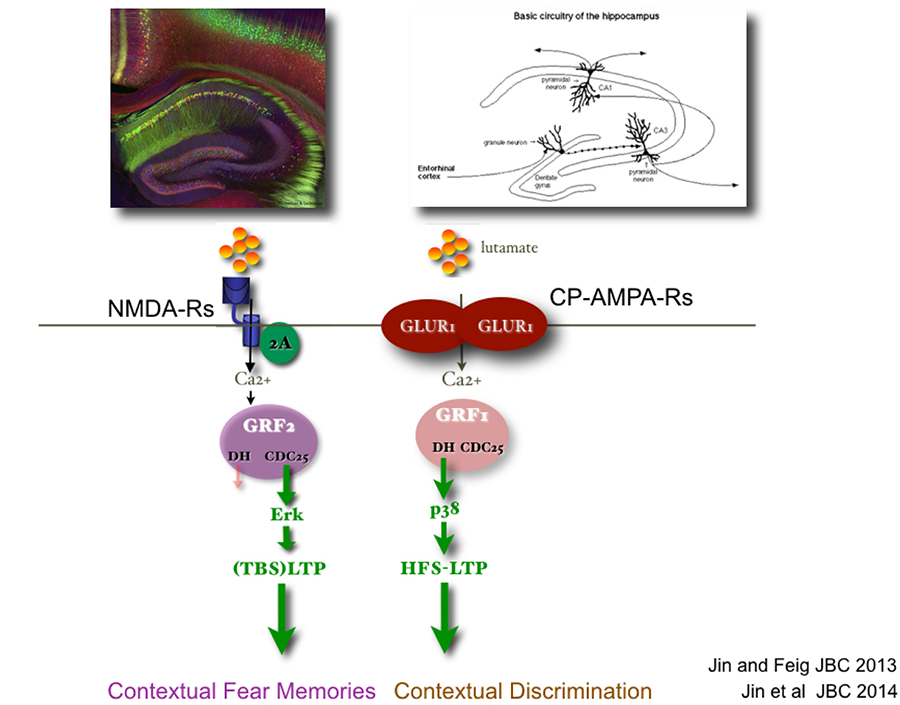
Ras proteins are activated by a family of nucleotide exchange factors. My lab discovered the calcium- activated Ras-GRF family of exchange factors (see Fig. 1). We showed previously that related Ras-GRF1 and Ras-GRF2 proteins, contain CDC25 and DH domains that activate Ras and Rac GTPases respectively. We also demonstrated that they play important but distinct roles in synaptic plasticity in the hippocampus, and learning and memory in post-adolescent male and female mice (see Fig. 2).
Figure 2. Ras-GRF1 and Ras-GRF2 are related GEFs expressed in hippocampus neurons.
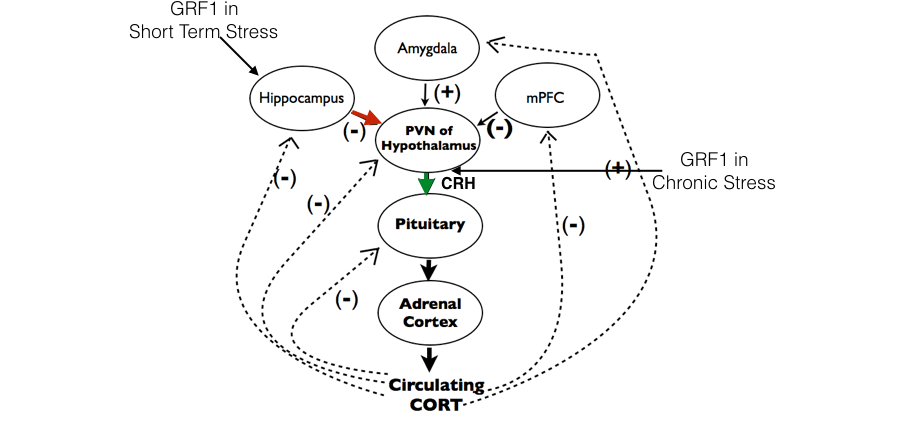
More recently, we have shown that Ras-GRF1 also controls the response to psychological stress through two different mechanisms. First, it contributes to the negative feedback role of the hippocampus in controlling the Hypothalamus, Pituitary, Adrenal (HPA) axis after short-term stress (Uzturk et al 2015). We have now found that after chronic stress Ras-GRF1 begins to contribute to the positive function of CRH cells in the Paraventricular Nucleus (PVN) of the hypothalamus in promoting corticosterone production in response to stress (see Fig. 3).
Figure 3. Fig. 3. Ras-GRF1 contributes to the HPA axis only in adolescent females through distinct functions in the hippocampus and hypothalamus
Remarkably, both of these Ras-GRF1 functions are displayed in adolescent females, but not in their adolescent male or adult female counterparts. At present we are focusing on revealing the genes regulated by Ras-GRF1 that carry out these age and sex-specific functions. Because distorted HPA axis function is thought to contribute to a variety of psychiatric diseases, a full understanding of Ras-GRF1 function in this context may be required to adequately treat females suffering from stress-associated disorders that begin during adolescence.
Transgenerational Inheritance of Anxiety and Defective Social Interactions Induced by Adolescent Exposure to Social Instability
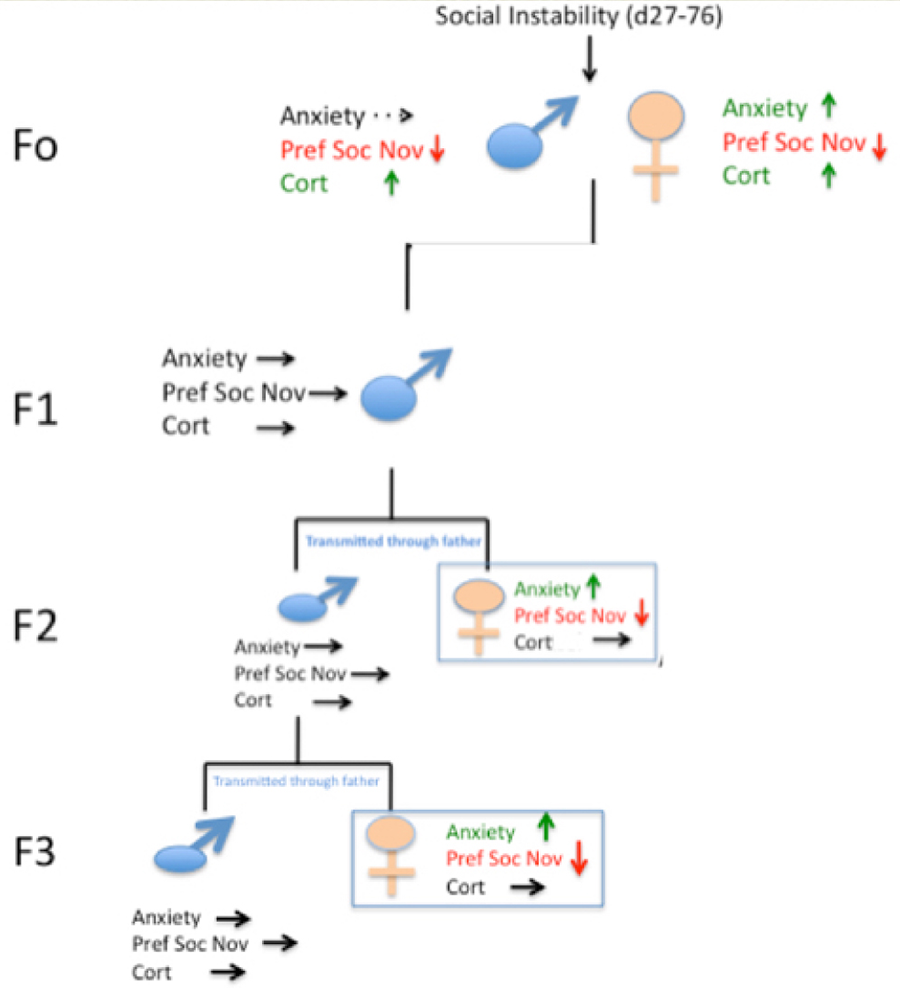
Figure 4 below shows that after males are exposed to social instability stress they transmit both elevated anxiety and defective social interactions (preference for social novelty) to their female offspring. Interestingly, even though male offspring do not display these altered behaviors they still pass on these defects to their female offspring across at least 2 more generations (see Saavedra-Rodriguez and Feig 2013 as well as the commentary by Champagne 2013).
Figure 4. Inheritance patterns of social instability.
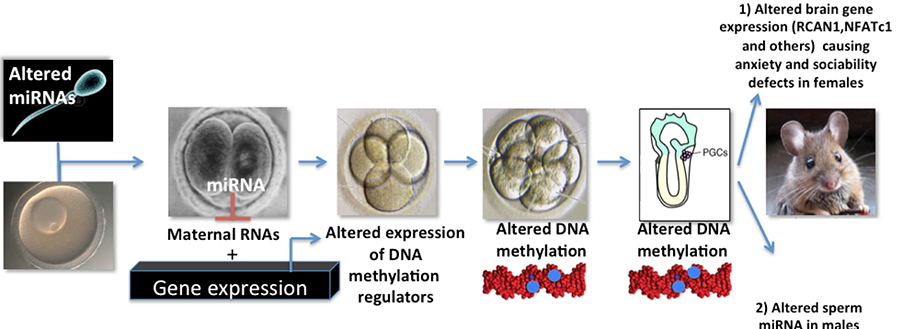
We are presently testing how stress-induced changes in sperm miRNA levels we detect in sperm from stressed males and their male offspring contribute to this phenomenon (see Fig 5 below)
Figure 5. Model for how changes in stressed-induced changes in sperm miRNAs may lead to abnormal behavior in F1 females and transmission of these traits to the F2 generation.
Finally, we have recently reported that early life exposure of men to abusive and/or dysfunctional families leads to the same sperm miRNA changes when they are adults as we detect in mice exposed to adolescent social instability stress. In mice, the stress effect on sperm miRNA content is transmitted to early embryos after fertilization and to sperm of F1 male offspring derived from them, along with elevated and anxiety and sociability defects to F1 females (see above) (Dickson et al, 2018). New human studies are underway testing idea that these sperm miRNA changes and stress associated behaviors are also transmitted to offspring of stressed men.