The Jonathan Garlick Lab
Bioengineering Human Tissues to Study Stem Cells, Cancer and to Fabricate Tissue Platforms for Drug Discovery
The Garlick lab performs studies at the interface of tissue engineering and stem cell biology. We use 3D, skin-like tissues constructed from cells derived from pluripotent stem cells to study their behavior in a biologically-relevant tissue context in ways that will inform their future therapeutic application.
Towards regenerative medicine and human therapeutics using human Induced Pluripotent Stem Cells (iPSC) cells
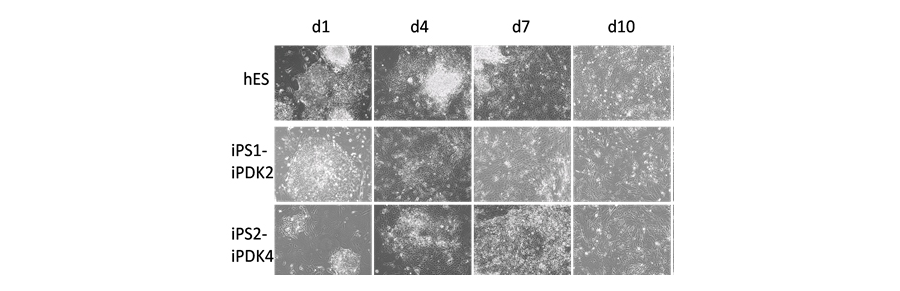
Our goal is to develop efficient ways to derive functional cell types form induced pluripotent stem (iPSC) cells and to use them to generate 3D tissues that demonstrate a broad range of biological functions. Our long term goal is to develop iPSC-derived cells for human therapies to replace or regenerate human cells and tissues that will restore normal function. We have shown that iPSC can differentiate towards stable fibroblast lineage fates (Figure 1) and that these cells demonstrate properties of human fibroblasts when grown in 3D skin-like tissues. We are currently studying how epigenetic regulation acts to control iPSC differentiation and if 3D tissue architecture predicts how these iPSC-derived cells may behave in in vivo after future transplantation.
Figure 1. ESC and iPSCs differentiation to fibroblast fate. ESC and iPSC were differentiated and monitored at various stages of differentiation. Representative images show the morphology of ESC and 2 iPSC lines after days 1, 4, 7, 10, 14, 21 and 28 of differentiation. Early morphologic changes showed differentiation beginning at the periphery of colonies (day 1). At later stages cells acquired fibroblast features of elongated, stellate cells (day 10 at days 21 and 28 of differentiation
Tissue Engineering meets iPSC-derived cells: From “Disease in a Dish” to “Disease in a Tissue”
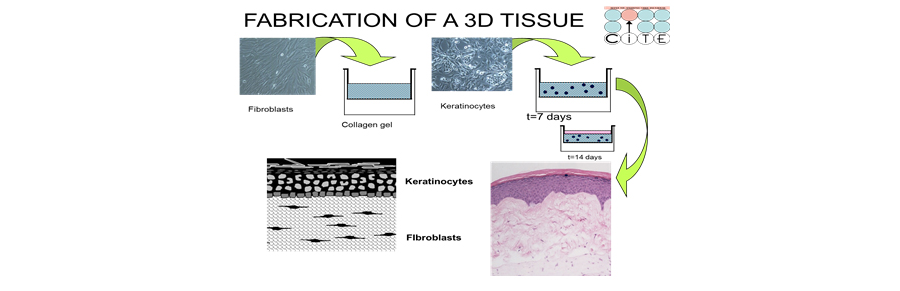
We have developed 3D skin-like tissues, known as human skin equivalents (HSE), that mimic the form and function of human skin. By growing these HSEs at an air-liquid interface on a connective tissue harboring fibroblasts, we can engineer fully differentiated, skin-like tissues displaying normal tissue architecture (Figure 2). We use iPSC that have been reprogrammed directly from patient tissues to study the properties of cells upon their differentiation from iPSC. We incorporate these iPSC-derived fibroblasts into 3D tissues to assess their biological potential and better understand how these cells integrate in these complex tissue microenvironments in ways that will better predict how they may behave after in vivo transplantation. By incorporating iPSC-derived cells that manifest specific disease phenotypes into our 3D, skin equivalent tissues, we can shift from conventional monolayer cultures (“Disease in a Dish”) to sophisticated 3D tissues (“Disease in a Tissue”).
Figure 2. Fabrication of three-dimensional tissue construction. (A) A collagen gel embedded with human dermal fibroblasts is layered onto a polycarbonate membrane. (B) After dermal fibroblasts contract and remodel the collagen matrix, keratinocytes are then seeded onto it to create a monolayer that will form the basal layer of the tissue. (C) Tissues are raised to an air-liquid interface to initiate tissue development that mimics in vivo skin
Studying Epigenetic Signatures Linked to Chronic, Diabetic Ulcers: Can iPSC reprogramming “rejuvenate” cell phenotypes to improve wound repair?
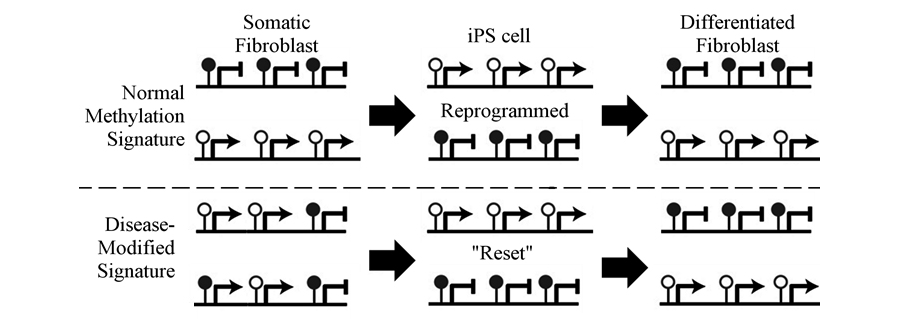
We have recently shown that the step-wise differentiation from pluripotency to mature fibroblast is accompanied by an improved biological potency. This suggests that normal skin fibroblasts can augment their function following reprogramming to iPSC and subsequent differentiation to fibroblast fate. We are now studying if acquisition of this improved biological potential can be used to treat complex diseases. Our lab is studying if reprogramming of fibroblasts from chronic diabetic ulcers to iPSC and their differentiation to fibroblasts, can improve their ability to heal chronic wounds. We are studying epigenetic mechanisms that control this augmented biological potential to improve treatment of these chronic disease states. For example, chronic wounds do not undergo proper healing due to methylation changes that cause the aberrant expression of genes needed for wound repair. Since DNA methylation marks are reset during reprogramming, it may be possible to modify epigenetic control of genes linked to a “repair-deficient” phenotype in wound-derived fibroblasts that can be reverted to “normalize” their cellular phenotype upon subsequent differentiation from iPSC to generate “repair-competent” fibroblasts (Figure 3).
Figure 3. Schematic model for reversing changes in DNA methylation due to chronic wound environment. Normal Methylation Signature - Somatic fibroblasts have a characteristic methylation signature that consists gene promoters that are methylated and repressed (e.g. OCT-4), as well as other promoters that are unmethylated and expressed (e.g. PDGFRβ). Following reprogramming, these methylation marks are reversed, and upon differentiation they are re-established to resemble the original somatic fibroblasts. Disease-Modified Signature -In a diseased state there are changes in methylation that allow expression of repressed genes and repress genes that should be expressed, resulting in aberrant phenotypes. Upon reprogramming, methylation is “reset” to allow for differentiation to fibroblasts with a normalized epigenetic signature.
The Biology of Adult Skin-derived stem cells
A central research area in our lab is the engineering of complex, 3D human skin, oral mucosa and other stratified epithelial tissues. Our goal is to optimize the potential of human adult and neonatal epithelial stem cells to generate these tissues. We study how stromal-epithelial interactions and the basement membrane niche direct the morphogenesis, survival, growth and differentiation of 3D tissues and how the fate of these stem cells is modulated. The long-term goal of these efforts is to fabricate human tissues in which predictable cell and tissue responses will lead to functional, stable tissues. This understanding will provide an important basis to determine gene regulatory networks and signaling pathways that modulate normal morphogenesis.
Translational Tissue Engineering: Human, 3D Tissue Platforms for Product Development and Drug Screening
We have adapted 3D tissues that mimic skin to provide tissue-based platforms that offer more reliable correlations between in vitro studies and in vivo outcomes during human clinical drug trials or when products are used. In this way, we use 3D tissues as a “pre-clinical” setting to accelerate development of drugs, potential therapeutics and skin-care products. Through our Center for Integrated Tissue Engineering , we facilitate the screening, pharmaceutical design and therapeutic application of lead candidate targeted to treat human disease and offer help to many skin care companies with the development of new products. In this way, we are playing a pioneering, strategic role is moving discovery and product development into this new dimension by providing a novel focus on clinical relevance using tissue engineering approaches.