The Jonas Galper Lab
Lipid Metabolism and Cardiovascular Signaling
The Galper lab focuses on the role of lipid metabolism in the regulation of cell signaling in the heart and vasculature and secondary effects of diabetes on the heart with the added emphasis on gender specific effects on the heart. He is studying the molecular basis for cardiac risk factors and the relationship between lipid lowering, hypertension and angiogenesis, both in an in vivo model for tube formation by endothelial cells, and in a mouse model for abdominal aortic aneurysm. He is also studying the role of lipids, GTP binding proteins and the regulation of gene expression by sterol responsive transcription factors and TGFß in the control of the autonomic response of the heart and the development of arrhythmias in both cell culture and mouse models. Most recently, he has extended these studies to gender specific effects in the development of cardiac dysfunction in diabetes. His clinical studies focus on the role of lipid lowering in regulating the autonomic response of the heart in patients treated with statins and its relationship to the development of cardiac arrhythmias and diabetic autonomic neuropathy.
GTP Binding Proteins and Cardiac Physiology
We have had a long standing interest in the role of GTP binding proteins in the regulation of cardiac physiology during cardiac development, in response to innervation of the heart, in response to growth factors and in the diabetic state. We developed an in vitro model for the study of the effects of lipid lowering on cardiomyocyte physiology. We made the surprising observation that lipid lowering markedly increased the response of atrial myocytes to parasympathetic stimulation. This increase in responsiveness was associated with an increase in the expression of the genes coding for proteins which play a role in the parasympathetic response pathway in the heart: the M2 muscarinic receptor; the heterotrimeric G-Protein, Gαi2; and the inward rectifying K channel, GIRK1/GIRK4. We demonstrated that this increase in gene expression was dependent on the activation of the small GTP binding protein Ras and demonstrated a new lipid dependent mechanism for the regulation of the activity of Ras and other small GTP binding proteins. Current studies focus on the mechanism for lipid regulation of Ras and Rho dependent kinases and their role in regulating expression of genes involved in signaling in the heart and vasculature via an effect on sterol responsive transcription factors.
Lipid Metabolism and the Autonomic Response of the Heart
Based of the fact that lipid lowering regulates the expression and function of sterol responsive transcription factors, we are investigating the interaction of these transcription factors and small GTP binding proteins in regulating specific downstream kinase pathways that control expression and function of genes involved in the response of the heart to autonomic stimulation using cultured myocytes and EKG and electrophysiology studies in genetically manipulated mice.
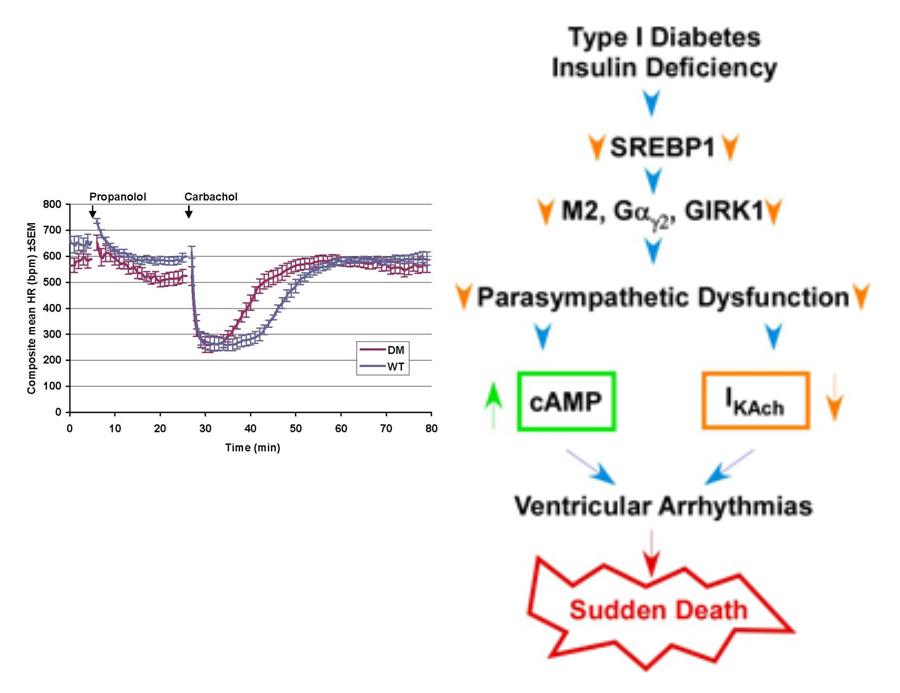
Figure 1. The role of sterols in the regulation of autonomic response of the heart and the development of ventricular arrhythmias. (Left) Diabetic mice (DM) and wild type animals were treated with carbachol and heart rate monitored with implanted EKG transmitters. The duration of heart rate slowing (braydycardia) is decreased and the rate of recovery of heart rate is increased in the DM animals compared to WTanimals. (Right) Schematic representation of proposed role of sterol sensitive transcription factors in the regulation of expression of genes involved in the autonomic response of the heart, effects on ion channels and the development of ventricular arrhythmias.
Furthermore given the role of insulin in the regulation of sterol responsive transcription factors, we have demonstrated that in a mouse model for diabetes, hypoinsulinemia is associated with a decrease in the expression of genes which regulate the response of the heart to parasympathetic stimulation. Since the parasympathetic responsiveness of the heart plays a role in the protection of the heat from arrhythmias, the long term goal of these studies is to determine the relationship between the regulation of sterol responsive transcription factors and the genesis and treatment of arrhythmias. One of the new directions of these studies is to determine the role of insulin and the PI3Kinase/Akt/GSK3ß pathway in the regulation of the autonomic response of the heart via the regulation of sterol responsive transcription factors.
Effect of Lipid Lowering by Statins on the Parasympathetic Response of the Heart and the Development of Arrhythmias
Since the parasympathetic responsiveness of the heart plays a role in the protection of the heat from arrhythmias, the long term goal of these studies is to determine the relationship between the regulation of sterol responsive transcription factors and the genesis and treatment of arrhythmias. These studies represent a direct extrapolation of our in vitro observations on the role of lipids in the regulation of the parasympathetic response. Studies include the effect of statins and lipid lowering on ventricular ectopy and arrhythmias in mice. Also in progress is a double blind crossover study of patients randomized to simvastatin and Pravastatin using heart rate variability analysis to determine effects on parasympathetic responsiveness as well as measurements of cardiac ectopy. Direct studies of the effects of statins and lipid lowering on arrhythmias and diabetic autonomic neuropathy are planned.
Cholesterol Metabolism, Angiogenesis and Abdominal Aortic Aneurysms (AAAs): The Role of Statins
We have demonstrated that statins regulate angiogenesis both in vivo and in vitro via the control of the activity of the small GTP binding protein Rho. Our current studies deal with the relationship between hypertension, hyperlipidemia and angiogenesis and the role of statins in regulating the development of abdominal aortic aneurysms in a mouse model. More recently we have initiated studies of the role of innate immunity and effects on the distribution of T-cells to the abdominal aorta in the development and progression of AAAs.
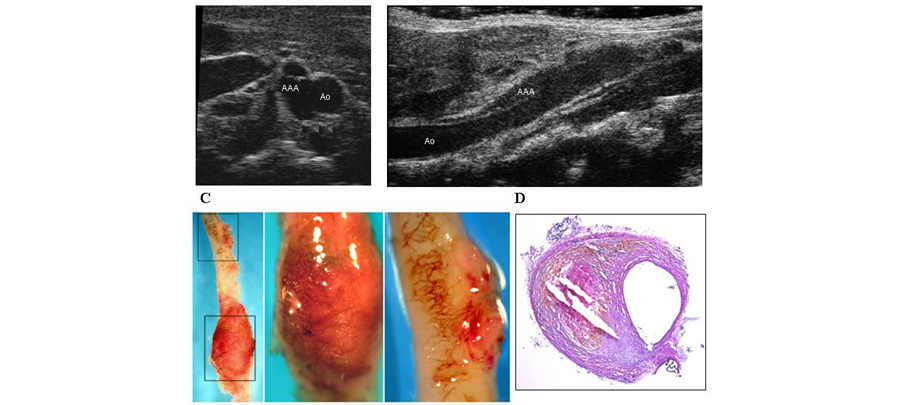
Figure 2. Abdominal aortic aneurysm induced by Angiotensin II in an ApoE-/- mouse. A, B, representative ultrasound images in the transverse (A) and longitudinal (B) planes. C, Representative images of whole-mount staining demonstrating neovascularization of the aneurysms at high power. D, Representative photomicrograph of aortic cross section stained with hematoxylin and eosin.