The James Schwob Lab
A Simple but Powerful Neural System for Understanding Neurocompetent Stem Cells and Analyzing Neurogenesis
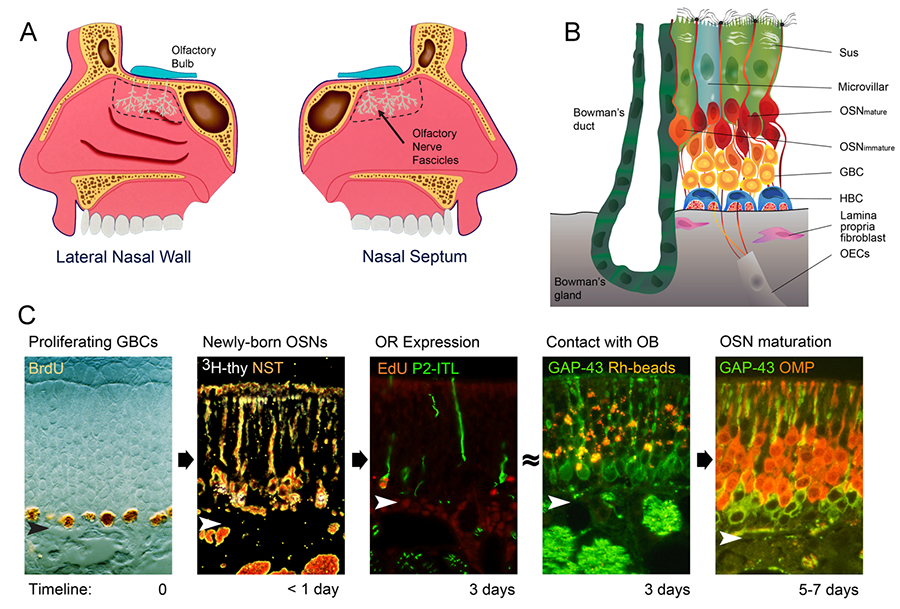
The challenges of achieving a mechanistic understanding of the development of the nervous system and its recovery (or not) after injury require a focus on systems and models that offer technical advantages by being simpler (for example, simple organisms like C. elegans or D. melanogaster, or simple models, such as tissue culture) yet sufficiently complex to be broadly relevant. Our lab’s approach focuses on a specific part of the mammalian nervous system, namely the peripheral part of the olfactory system – the neural substrate for perceiving and relaying smell stimuli – as a particularly powerful model for studying neural development and regulation. The peripheral olfactory apparatus consists of the olfactory epithelium, the specialized neuroepithelium that forms the lining of the posterodorsal part of the nasal cavity (Figure 1), and the axonal projection from that neuroepithelium onto the olfactory bulb, the part of the brain that serves as the first CNS relay for smell information.
Figure 1. (A) The olfactory epithelium lines the posterodorsal nasal cavity immediately inferior to the cranial cavity. (B) The normal epithelium is composed of a handful of cell types: sustentacular cells (Sus), microvillar cells (a supporting cell variant), olfactory sensory neurons – both mature and immature (OSNs), globose basal cells (GBCs), horizontal basal cells (HBCs), Bowman’s duct and gland cells. Deep to the basal lamina, the fascicles of the olfactory axons are ensheathed by the specialized glia of the olfactory nerve, the olfactory ensheathing cells (OECs). In addition, stromal cells (fibroblasts) of the lamina propria secrete signals that regulate epithelial assembly and turnover. (C) Remarkably, neurogenesis carries on throughout life and the process by which neurons are born and differentiate occur with a fixed time schedule, including the expression of olfactory receptors (ORs).
Why is the peripheral olfactory system such a powerful model for neural development? The neurons of the epithelium are in direct contact with the airborne environment (in order to transduce odorant stimuli), which makes them vulnerable to injury. Because the sense of smell is absolutely essential for survival and reproduction in many mammals (and is fundamental to health and nutrition in humans), the epithelium retains a life-long capacity to regenerate both the neurons and the non-neuronal populations. Furthermore, the newly born neurons retain a capacity to reinnervate the olfactory bulb, and reinnervation will restore sensory function. This regenerative capacity derives ultimately from the preservation of neurocompetent stem cells within the epithelium and their enhanced activation following injury.
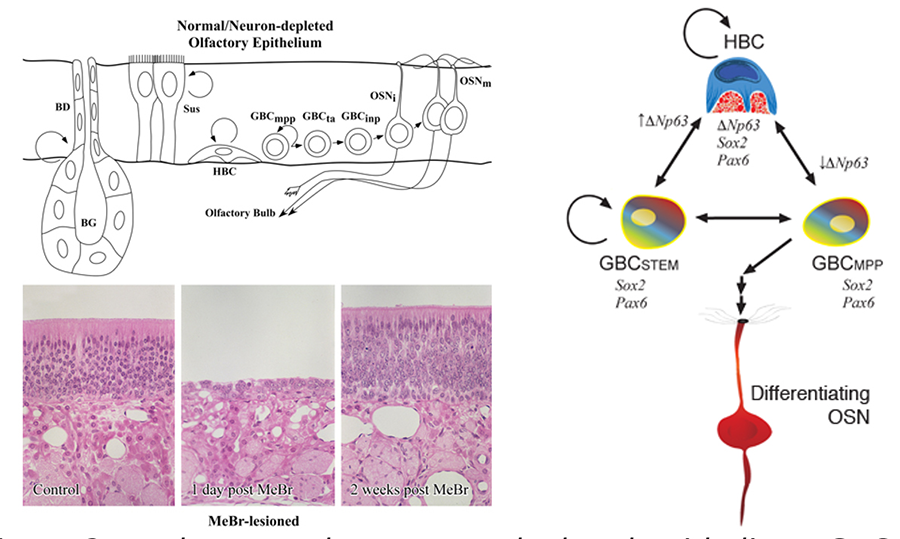
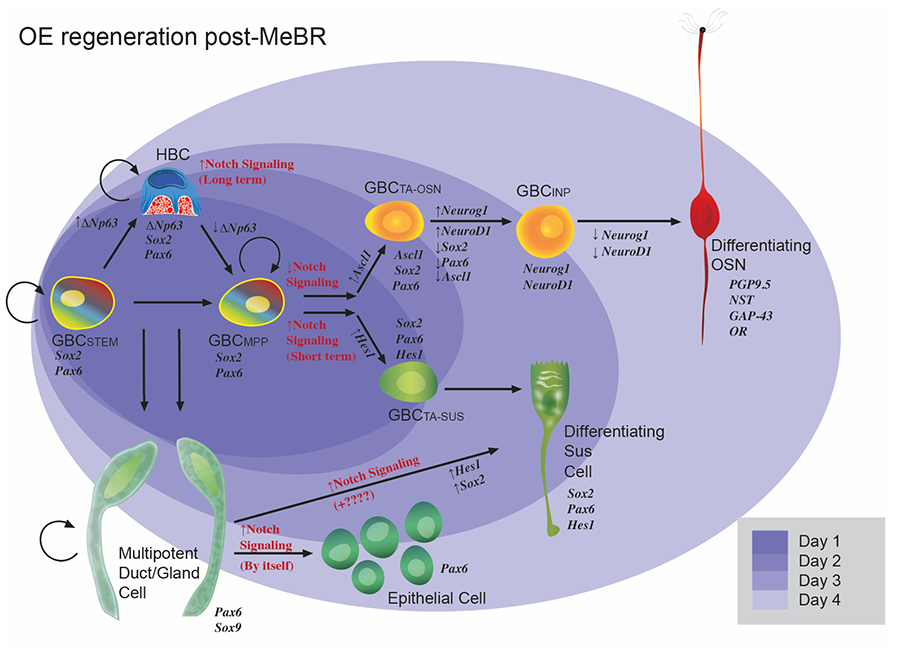
Figure 2. In the normal or neuron-depleted epithelium, GBCs go through a series of stages during the progressive differentiation into neurons; some are multipotent progenitors (mpp), transit amplifying GBCs (ta) are downstream, while others are immediate neuronal precursors (inp) whose daughters exit the cell cycle and become neurons. Destruction of the mature cells of the olfactory epithelium by exposure to methyl bromide gas (MeBr), a selective olfactotoxins, activates a repair program that regenerates the epithelium, including its neuronal population within a few weeks.
As a consequence of these remarkable features, the olfactory periphery offers significant analytical advantages: the birth of new neurons can be synchronized by experimental injury (Figure 2), the synchronized population is very large (which facilitates biochemical analyses), the epithelium can be manipulated by transplantation of stem and progenitor cells (Figure 3) or retroviral transduction in vivo, and assemblies of epithelial cells with a 3-D architecture in tissue culture retain the multipotency to engraft after transplantation and participate in the recovery of the epithelium (Figure 4), which makes their formation a useful assay for delineating growth factor regulation of epithelial biology with a moderate throughput capacity.
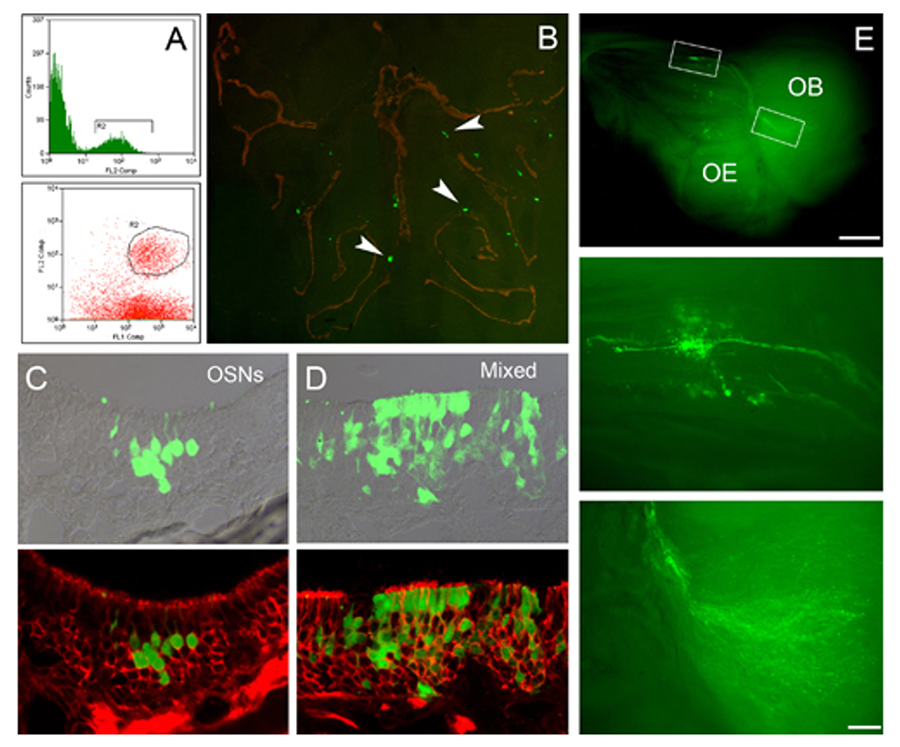
Figure 3. Transplantation is an analytic paradigm for investigating the differentiative potency of the various cell types of the epithelium. (A) FACS is used to isolate GBCs (circled), (B) which engraft into the epithelium and give rise to clones of donor-derived cells. (C) GBC-derived clones encompass a broad range of outcomes: clones can be composed of only neurons (OSNs) or Sus cells (not shown) or (D) mixed cell types, which can include neurons, Sus cells, gland/duct cells, basal cells, or respiratory epithelium, all of which derive from a common embryonic precursor. (E) Clones of donor-derived neurons extend axons from the olfactory epithelium (OE) to the olfactory bulb (OB) where they terminate.
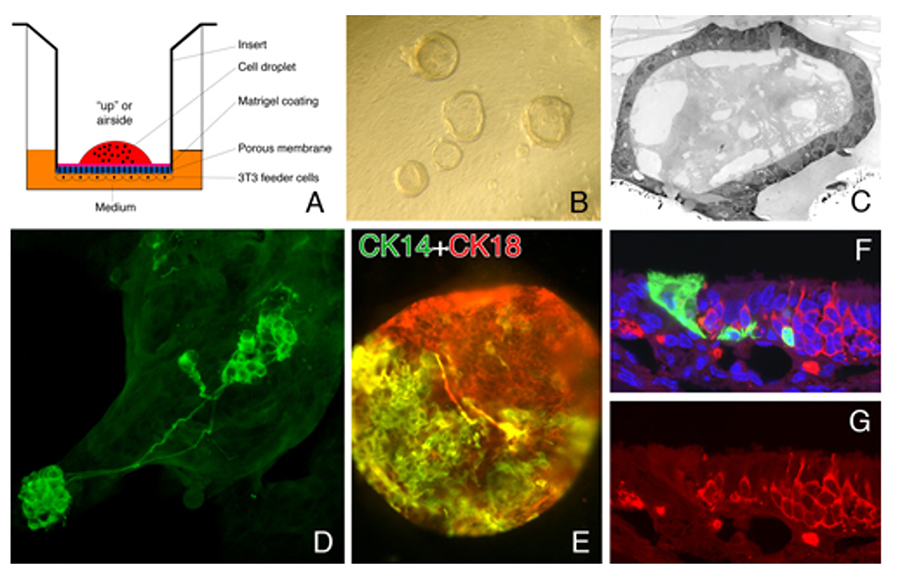
Figure 4. Cells from the olfactory epithelium form epithelioid spheres when cultured at the air-media interface on top of feeder cells from the lamina propria. (A) Schematic of culture. (B) Spheres resting on insert. (C) EM of hollow sphere. (D) Neurons that differentiate in spheres closely resemble the ones observed in the intact tissue. (E) Cells with HBC (CK14) and sus cell (CK18) markers form within the spheres. (F, G). Sphere-derived cells engraft after transplantation and give rise to the full panoply of cell types (Jang et al, 2008).
A detailed understanding of all of these features is a necessary precondition for the therapeutic use of the easily accessible neurocompetent stem cells of the olfactory epithelium either in the nose or elsewhere.
By exploiting these advantages, we have made two key advances. First, we and others have demonstrated that there are two populations of multipotent stem-like cells in the adult olfactory epithelium: globose basal cells (GBCs) and horizontal basal cells (HBCs) (Figures 2, 5).
Figure 5. Schematic illustrating the current model of epitheliopoiesis in the OE during recovery from methyl bromide (MeBr) injury, which is substantially more complex than the progression observed in the normal OE (cf., Figure 2). The various subsets of GBCs differ in their differentiative capacity. The rainbow gradient filling the GBCSTEM and GBCMPP symbolizes their active multipotency, i.e., those GBCs that generate, in aggregate, all of the epithelial cell types following epithelial injury, including HBCs. Stem GBCs are likely to be functionally equivalent to the cells of the embryonic olfactory epithelium, but largely quiescent in the normal adult epithelium. Mulitpotent (MPP) GBCs can be driven to a non-neuronal fate by Notch signaling, and to neurons when Notch signaling is inhibited. HBCs are a reserve population that are late to develop but can be called upon to help with epithelial reconstitution. HBCs differentiate from GBCs around birth, when the epithelium become vulnerable to the extrauterine environment.
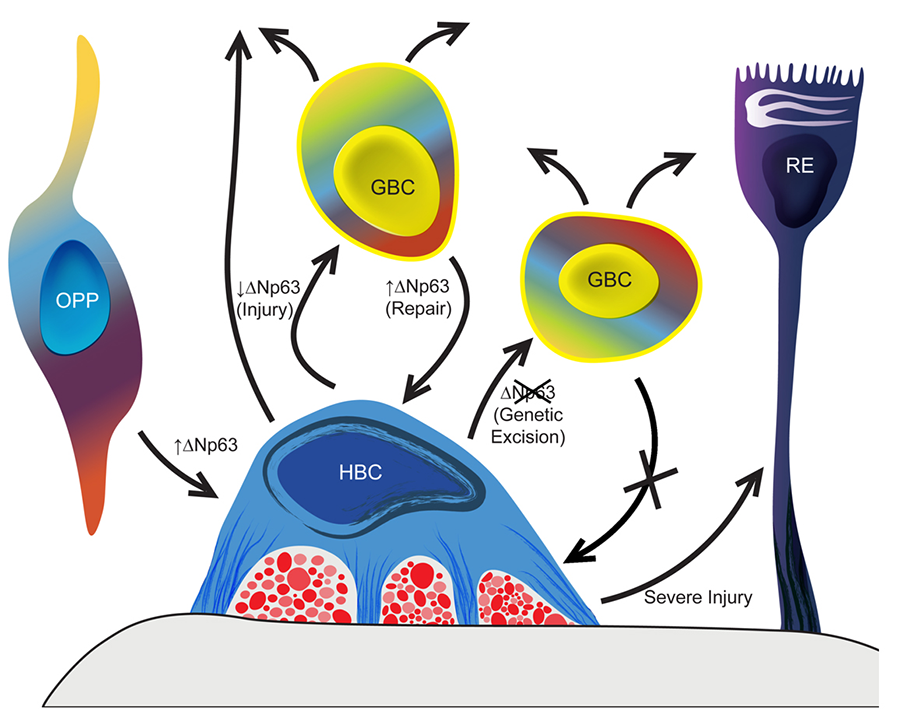
GBCs emerge early in embryonic development and give rise to HBCs around the time of birth. The HBCs are a kind of reserve population that requires activation by injury to assume their multipotency. We have demonstrated that the activation of the HBCs from dormancy requires the down-regulation of the transcription factor ΔNp63 and that its down-regulation is sufficient to activate the HBCs (Figure 6).
Figure 6. p63 is the master regulator of the formation and activation-dormancy of the HBCs – the second category of olfactory epithelial stem cells, which are held in reserve normally. In the absence of direct epithelial damage HBCs are dormant – mitotically quiescent and non-participatory in the generation of replacement neurons. With injury, ΔNp63 levels decline and many of the HBCs differentiate into GBCs, which in turn give rise to neurons and other epithelial cell types.
In contrast, The GBC population is heterogeneous with respect to the differentiative capacity of different GBC subtypes defined by their transcription factor profile (Figure 5). Some among the GBCs – those expressing Sox2 and lacking the neurogenic basic helix-loop-helix factors – are multipotent even in the absence of injury. Others are normally fated to make neurons, but they can be driven back “upstream” in the progenitor cell hierarchy (Figure 7). That dedifferentiation depends on Sox2 and is opposed by the polycomb repressor complex.
Figure 7. On the left: minimal development after transplant from normal epithelium. Right: transplanted cells generated all cell types of the nasal tissue when harvested after injury.
Future experiments will be targeted toward identifying and characterizing in detail the stem and progenitor cell subpopulations of the GBCs, and defining the cues that emanate from the stromal tissue deep to the olfactory epithelium and the epithelium itself that regulate progenitor cell function in vivo and in vitro. The translational implications have to do with repairing the senescence of the olfactory epithelium and the accompanying loss of smell function by regulating, redirecting and replacing olfactory epithelial stem cells that no longer function properly.