The Iris Jaffe Lab
Molecular Mechanisms Important in Cardiovascular Disease
The Jaffe laboratory is a focused on understanding the molecular mechanisms by which the vasculature becomes dysfunctional to lead to common cardiovascular conditions including heart attack, stroke, high blood pressure, in-stent restenosis, vein graft failure, heart failure and pulmonary hypertension. A major area of interest is the role of the hormone aldosterone and the receptor by which it functions, the mineralocorticoid receptor (MR), in the molecular mechanisms of cardiovascular disease. The steroid hormone aldosterone is the final step in the renin-angiotensin-aldosterone pathway that regulates blood pressure and electrolyte homeostasis by activating MR in the kidney to regulate genes involved in renal sodium handling.
We and others have demonstrated that MR is expressed in cells of the human blood vessel supporting the possibility that direct effects of aldosterone on the vasculature could play a role in vascular function and disease. This is clinically significant because in human clinical trials, pharmacologic inhibition of the MR prevents heart attacks, strokes, and cardiovascular deaths with minimal effects on systemic blood pressure. We are exploring the molecular mechanisms for these clinical findings in order to gain a better understanding of the mechanisms of cardiovascular disease and to identify novel therapeutic drug targets for common vascular diseases. The Jaffe laboratory routinely use in vitro molecular and cellular biology methods, primary human vascular cell culture models, state-of-the-art transgenic mouse models with in vivo capabilities to study rodent models of cardiovascular physiology and disease, unbiased transcriptomics, proteomics, and epigenomics methods, and translational studies utilizing human samples from our patients at Tufts Medical Center.
The role of vascular MR in endothelial dysfunction, atherosclerosis, and vascular remodeling
We have demonstrated that MR is expressed in human vascular cells where it acts as a ligand-activated transcription factor to regulate gene expression in response to the hormone aldosterone. In human vascular smooth muscle cells, we have found that MR regulates genes involved in vascular fibrosis and calcification.
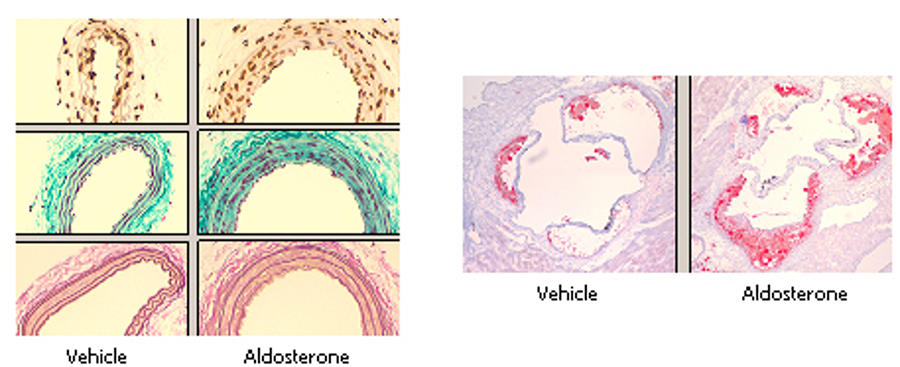
Figure 1. Animal models of aldosterone-induced vascular injury and atherosclerosis used in the Jaffe lab to study the in vivo role of MR in vascular function and disease.
Sex differences in mechanisms of cardiovascular disease
We also study sex differences in mechanisms of cardiovascular diseases.
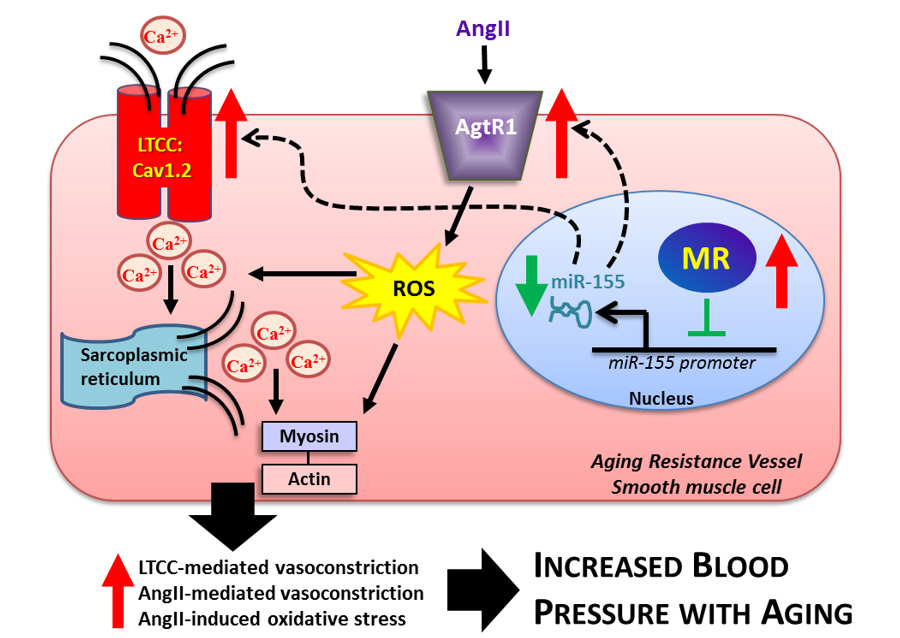
Mechanisms of vascular aging and hypertension
Learn more about this work in Dupont et al, 2016 and by reviewing the figure below.
Figure 2. Model of the Role of Smooth Muscle Cell Mineralocorticoid Receptor in Vascular Aging. The Jaffe lab has identified a mechanism contributing to vascular aging in which mineralocorticoid receptor (MR) expression rises in aging resistance vessel smooth muscle cells where MR suppresses miR-155 transcription leading to the up-regulation of angiotensin type 1 receptors (AgtR1) and L-type calcium channels (LTCC). The up-regulation of these pro-constrictive genes contributes to SMC calcium influx and reactive oxygen species (ROS) production. This enhances AngII-induced and LTCC-mediated vasoconstriction and vascular oxidative stress, components of vascular aging. Restoration of miR-155 in SMC in aging vessels reverses these changes with aging. These aging-induced alterations in vascular function ultimately contribute to increased blood pressure with aging. MR antagonists may decrease blood pressure in part by inhibiting this mechanism in the aging vasculature. Cav1.2 = voltage gated subunit of the L-type calcium channel, Ca2+ = calcium, AngII = angiotensin II.
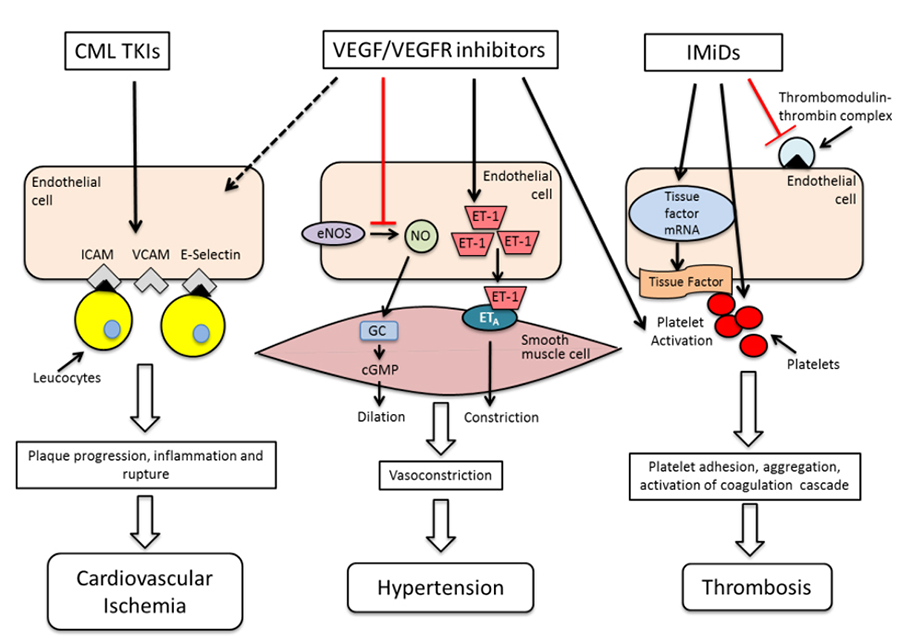
Vascular complications of targeted cancer therapy
Learn more about this work in Gopal, et al 2016 and by reviewing the figure below.
Figure 3: The endothelial cell as a central mediator of vascular toxicities of targeted anti-cancer agents. Mechanisms of endothelial cell dysfunction caused by targeted anti-cancer therapy include increased expression of leucocyte adhesion molecules, decrease in nitric oxide and increased endothelin-1 production, and increase in tissue factor expression leading to vascular inflammation, hypertension and thrombosis respectively. These mechanisms are often interlinked. Nitric oxide also serves as an anti-platelet and anti-inflammatory agent in addition to regulating vascular tone. VEGF: vascular endothelial growth factor, VEGFR: vascular endothelial growth factor receptor, NO: nitric oxide, ICAM: intercellular adhesion molecule, VCAM : vascular cell adhesion molecule, CML: chronic myelogenous leukemia, TKI: tyrosine kinase inhibitor, IMiDs: immunomodulatory agents.,cGMP: cyclic guanosine monophosphate, GC: guanylate cyclase, ET-1: endothelin 1.
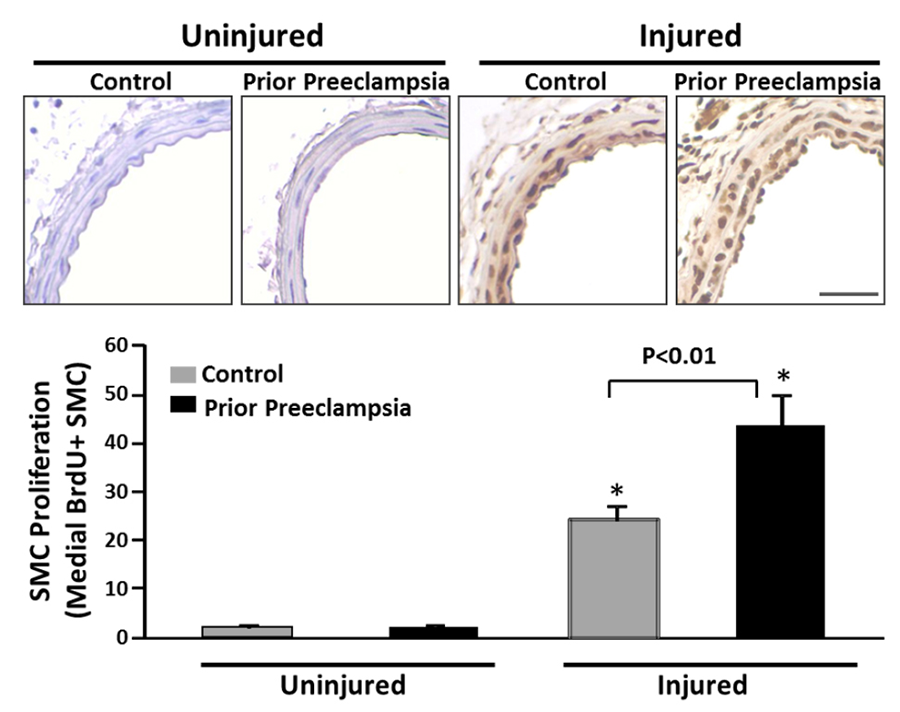
Pregnancy complications and future cardiovascular disease
Learn more about this work in Pruthi, et al, 2015 and by reviewing the figure below.
Figure 4: Enhanced smooth muscle cell proliferative response to vascular injury after prior exposure to PE. Two months after exposure to experimental preeclampsia (Prior Preeclampsia, black bars) or Control pregnancy (grey bars), mice were subjected to unilateral wire carotid injury along with bomodeoxyuridine (BrDU) infusion to label proliferating cells. Smooth muscle cell (SMC) proliferation was quantified fourteen days after injury in the uninjured and injured carotid arteries. Representative BrDU-stained sections are shown above. Bars below represent average number of medial BrDU positive smooth muscle cells. Scale bar=0.5 mm, N=6 Control, N=8 Prior Preeclampsia. *P<0.001 versus uninjured. P value is not significant for preeclampsia exposure within uninjured vessels.