The Gail Sonenshein Lab
ADAM8 Drives Triple-Negative Breast Cancer and is a Target for Treatment
Despite recent advances in cancer treatment, the World Health Organization reports that over 450,000 women worldwide die yearly of metastatic breast disease. Identifying new therapeutic options to effectively reduce breast cancer dissemination is crucial to improving survival of high risk patients, in particular women with Triple-Negative Breast Cancer (TNBC) that lacks targeted therapy. TNBC represent ~15% of all breast cancer cases, but accounts for more than 25% of breast cancer deaths due to lack of identified therapeutic targets, e.g., steroid hormone and HER2 receptors, and poor responsiveness to chemotherapy. We recently demonstrated ADAM8 is induced by a multistep pathway downstream of NF-κB, and drives the aggressive phenotype in breast cancer, in particular in TNBCs. Thus, our current focus is on ADAM8 and cancer.
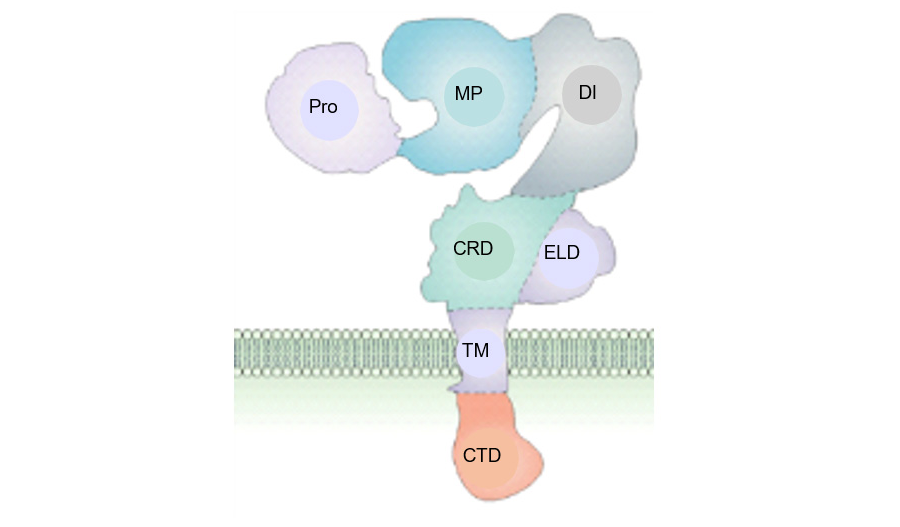
Synthesized as a 120 kDa proform, the transmembrane ADAM8 protein can dimerize or multimerize and autocatalytically clip off its prodomain, leaving an active membrane-anchored metalloprotease of ~90 kDa (Fig. 1). Active ADAM8 can be further processed by the release of the metalloprotease (MP) domain into the extracellular matrix, which leaves a remnant form within the membrane. Both active and remnant forms mediate cell adhesion predominantly through the disintegrin (DI) domain, notably by direct binding to integrins. ADAM8 was found to be non-essential under physiological conditions, as evidenced by normal development, lack of pathological defects, and normal lifespan of ADAM8 deficient mice. Interestingly, ADAM8 expression was detected under several pathological conditions characterized by inflammation, including cancer.
Figure 1. Scheme of ADAM8 structure. Pro: prodomain; MP: metalloproteinase; DI: disintegrin; CRD: cysteine-rich; ELD: EGF-like; TM: transmembrane; CTD: cytoplasmic domains. Proform (inactive): 120 kDa; Active (ADAM8 without Pro): 90 kDa; Remnant (Active without MP): 60 kDa. G Murphy, Nature Rev Can 2008 Modified.
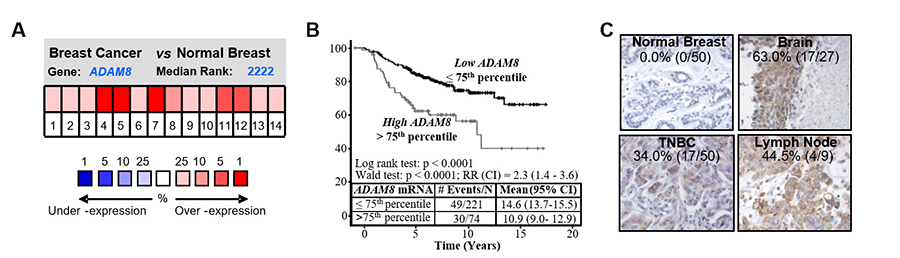
Interestingly, ADAM8 expression was detected under several pathological conditions characterized by inflammation, including cancer. In our recent paper on breast cancer (Romagnoli et al, 2014), ADAM8 mRNA levels were shown to be significantly higher in breast tumors, especially in basal-like tumors, known to be mostly TNBCs, and to correlate with a poor clinical outcome (Fig. 2A-B). Further, immunohistochemistry seen in Fig. 2C showed that 34% of TNBCs and 48% of breast cancer-derived metastases in patients display abundant ADAM8 levels whereas adjacent normal mammary tissue was negative.
Figure 2. ADAM8 is overexpressed in breast cancer. (A) Microarray data showing elevated ADAM8 mRNA levels in breast cancer vs normal breast tissue (P = 0.025, pool of 14 analyses, Oncomine. (B) Kaplan-Meier curve of overall survival for 295 patients with primary breast cancer stratified based on ADAM8 mRNA levels. (C) IHC analysis of ADAM8 expression in samples of primary TNBC tumors and adjacent normal tissue (left panels) and in breast cancer-derived metastases to the brain and lymph node (right panels). The percentage and cases with positive staining are indicated.
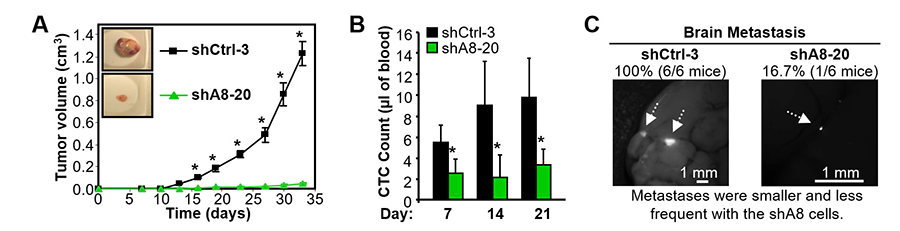
Knockdown of ADAM8 in MDA-MB-231 or Hs578T TNBC cells significantly decreased their ability to migrate, grow in an anchorage independent fashion or form invasive colonies in Matrigel. Stable ADAM8 knockdown in MDA-MB-231 TNBC cells profoundly reduced their ability to form tumors and metastasize in mice. Control (shCtrl-3) and ADAM8 knockdown (shA8-20) MDA-MB-231 TNBC cells were injected into the mouse mammary fat pad (MFP) of female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. After two weeks, the shCtrl-3 group started to develop mammary tumors that progressed rapidly. In contrast, shA8-20-derived tumors failed to grow beyond 0.05 cm3 after more than 4 weeks (Fig. 3A). In collaboration with Irene Georgakoudi’s lab, we showed that mice carrying shA8-20 tumors had significantly lower numbers of Circulating Tumor Cells (CTCs) throughout the time course, even at early time points when tumors were barely detectable in both groups (Fig. 3B), suggesting ADAM8 increases the risk for developing distant metastases. In fact, all of the animals implanted with shCtrl cells showed multiple brain metastases, while only one mouse was detected with a small metastasis in the shA8 group (Fig. 3C), which is consistent with the patient data. Solid tumors need to overcome hypoxic stress to grow beyond a palpable size. Increased ADAM8 levels were seen in shCtrl-3 but not shA8-20 cells under hypoxic conditions; strong ADAM8 staining was detected around the necrotic areas in shCtrl-3 tumors but absent in tumors derived from shA8-20 cells.
Figure 3. ADAM8 knockdown profoundly decreases tumor formation, angiogenesis and metastatic spread in an orthotopic mouse model. MDA-MB-231 derived shCtrl-3 and shA8-20 cells were injected into the MFP of female NOD/SCID mice (n = 7/group). (A) Tumor volume was measured twice a week (mean ± S.E.M.). The experiment was terminated when tumors in the control mice reached a size of ~1 cm3 as per the approved mouse protocol. (B) Blood drawn from 4 mice/group on the indicated days after tumor cell implantation into the MFP was subjected to flow cytometry to measure CTCs. (C) The presence of brain metastases was examined by fluorescent microscopy and the frequency of positive findings indicated above (n = 6/group). Only 1 of the 6 mice in the shA8-20 mouse cohort displayed a brain metastasis; the other 5 were negative. Representative photographs are shown. *P <0.05.
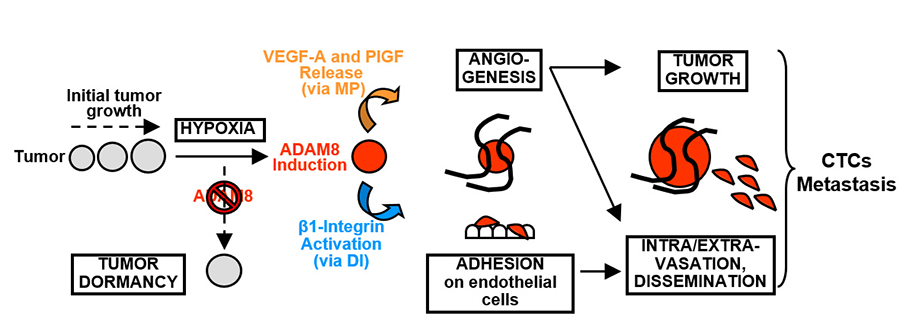
Mechanistic studies led to the model illustrated in Fig. 4. ADAM8 was responsible for release of pro-angiogenic factors (VEGF-A, PlGF and others) leading to induction of tumor angiogenesis and growth, and this was attributable to its protease activity (MP domain). Activation of β1-integrin needed for adhesion of cancer cells onto an endothelial layer and tumor dissemination was attributed to the DI domain of ADAM8. Strong β1-integrin activation was observed at the peritumoral area of control mammary tumors, but absent in shA8-20 tumors. Importantly, treatment with an anti-ADAM8 antibody that inhibited both its MP and DI activities reduced growth and metastatic dissemination of tumors derived from MDA-MB-231 TNBC cells. Thus, ADAM8 drives growth and metastasis of TNBC-derived tumors and is a promising target for an antibody-based therapy approach for these highly aggressive tumors.
Figure 4. Model of the roles of ADAM8 in breast tumor growth and dissemination. When solid tumors reach a few millimeters in diameter, hypoxic stress is induced, which leads to ADAM8 induction resulting in strong pro-angiogenic signaling, via release of VEGF-A and other pro-angiogenic factors into the extracellular compartment (via its MP activity), and endothelial cell recruitment. The ADAM8 DIS domain also promotes β1-integrin activation on tumor cells needed for their adhesion onto and transmigration through the blood vessel wall, which supports CTC dissemination and metastases. Importantly, if the induction of ADAM8 is blocked, there is an insufficient angiogenic response, leading to tumor mass dormancy or slowing of growth, and to a striking reduction of CTCs and metastases.
Based on these findings, work in our laboratory is currently focused on development and testing of various anti-ADAM8 antibody-based strategies for treatment of patients with ADAM8-positive cancers. In addition, we are pursuing studies to further elucidate the role of ADAM8 in cancer, including mechanisms of ADAM8 action in environmental carcinogenesis and resistance to chemotherapy, and the use of ADAM8 and downstream targets as markers of disease progression (Das, et al, 2016; Lyons, et al, 2016).