The Athan Kuliopulos Lab
Protease-Activated Receptors in Inflammation and Vascular Biology
Protease-activated receptors (PARs) are a unique class of G protein-coupled receptors that play critical roles in thrombosis, inflammation, and cancer. The PARs are cleaved by thrombin and other proteases at a specific peptide bond to expose a new N-terminus that binds to the body of the receptor in an unusual intramolecular mode. These receptors are widely expressed throughout human tissues including blood cells, endothelium, liver, kidney, lung, skin, and invasive cancer cells. A major interest of our research group is to study the molecular mechanism of protease activation of the PARs and the subsequent signaling in vascular cells and in cancer.
Figure 1. Interest in PARs and their role in physiology and disease has heightened in recent years.
PARs and Thrombosis
Thrombosis associated with the pathophysiological activation of platelets and vascular cells has brought thrombin and its receptors to the forefront of cardiovascular medicine. Protease signaling through the protease-activated receptors (PARs) has been shown to influence a wide range of physiological responses including platelet activation, intimal hyperplasia, inflammation, and maintenance of vascular tone and barrier function. A major goal of our Center of Hemostasis and Thrombosis Research is to determine the efficacy and safety of experimental anti-thrombotic agents in patients. We conduct Phase 1, Phase 2 and Phase 3 and translational studies which serve as the basis for improving the outcome, safety, and dosage of various drugs to most effectively treat patients with severe heart disease and myocardial infarction.
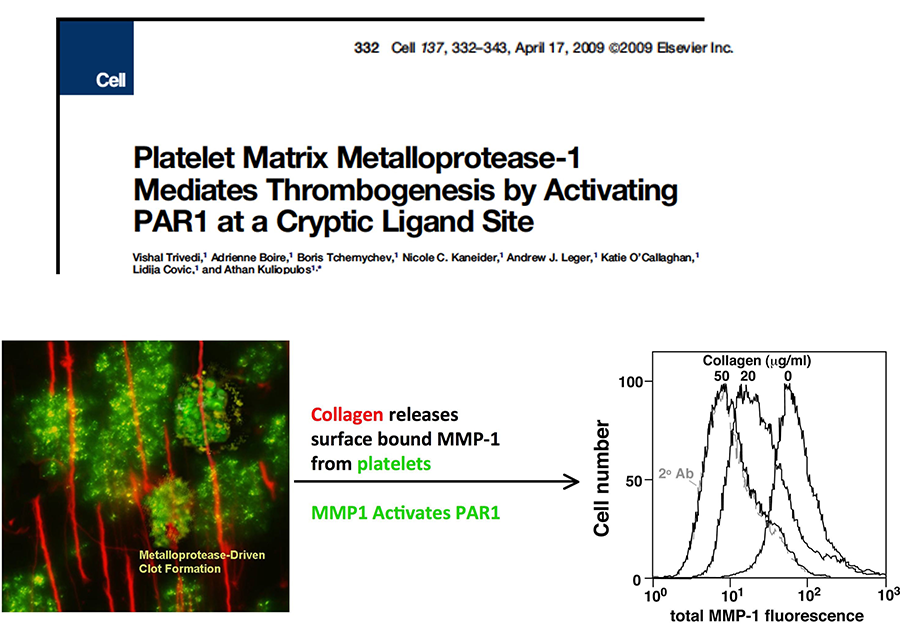
Emerging evidence also suggests that inappropriate matrix metalloprotease (MMP) activity may underlie the pathogenesis of atherosclerosis and vascular inflammation. Despite data that MMPs contribute to atherosclerotic lesion remodeling and poor outcomes, essentially nothing is known regarding the role of MMPs as active signaling molecules in controlling the behavior of vascular cells in the context of atherosclerotic disease. We recently made the unanticipated discovery that MMP-1 cleaves and activates protease-activated receptor-1 (PAR1) signaling in blood vessels. This is of major import as it was the first report of a direct signaling function of the principal collagenase in blood vessels. Notably, we found that MMP-1 activates PAR1 by cleaving the receptor at a distinct site from the canonical thrombin cleavage site which generates a longer tethered ligand that is biased towards a different spectrum of G protein signaling pathways. The studies in this project focus on the completely unexplored role of MMP1-PAR1 in the development of atherosclerotic plaques and understanding the pathophysiologic relevance of MMP1-PAR1 signaling on endothelium and macrophages. The mechanism linking these events to atherosclerotic plaque development will provide a framework to advance future therapies that could halt or reverse the progression of atherosclerosis.
Figure 2. The figure illustrates MMP1 activation of PAR1 on the surface of collagen (red)-stimulated platelets (green) under arterial shear conditions.
Pepducins as Novel Cell-Penetrating Intracellular Agonists and Antagonists of G Protein-Coupled Receptors
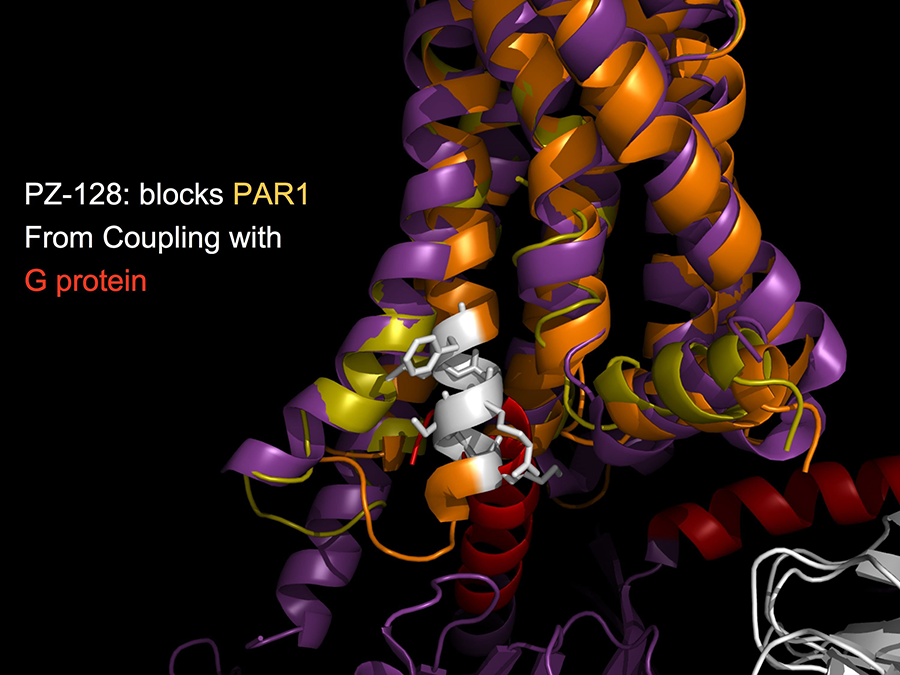
We have discovered and developed a new class of cell-penetrating, membrane-tethered peptides—named pepducins—that are based on the intracellular loops of receptors. Pepducins rapidly flip across the plasma membrane and cause full activation or inhibition of G protein-dependent signaling. Most remarkably, pepducins are selective for their cognate receptor and require receptor for activity both in cell culture and in animals. Drugs based on pepducins may prove to be the first therapeutically useful agents targeted to the receptor-G protein interface. Pepducins can also be used to define the roles of orphan receptors which lack known agonists or antagonists and can help define the complex pharmacologies of receptors whose functional interactions and signaling change over time. We are currently testing PZ-128 as a fast-acting PAR1 pepducin inhibitor in a multi-center phase 2 clinical trial which may confer significant clinical benefits to patients at high risk for life-threatening arterial thrombosis and myocardial infarction.
Figure 3. A model of pepducin PZ-128 (white) replacing its analogous i3 loop-TM6 helical segment of PAR1 (orange) to prevent coupling with G protein (red).