The Guo-fu Hu Lab
Ribonucleases in Cancers and Neurodegenerative Diseases
Angiogenin
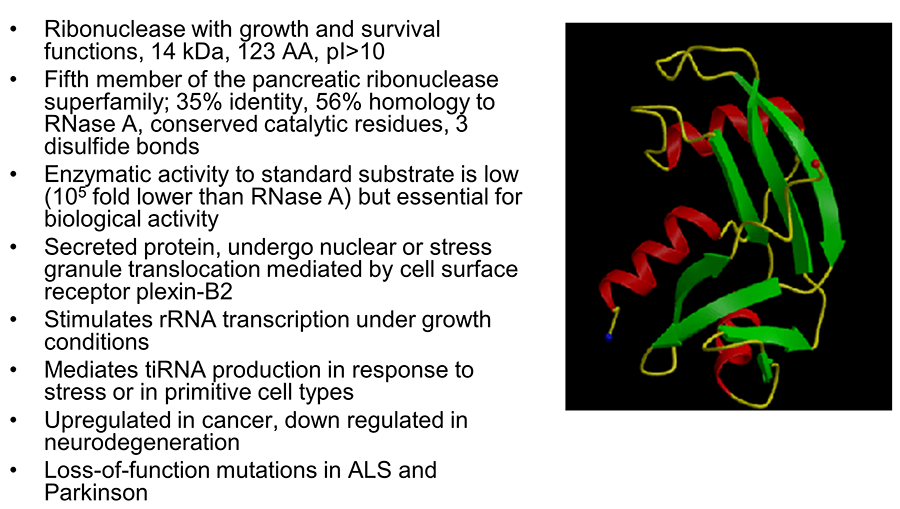
Angiogenin (ANG) was originally identified in 1985 as a tumor angiogenic factor based on its in vivo angiogenesis activities. The biological functions of ANG have since been extended from induction of neovascularization to regulation of cell growth and survival in a number of cell types including endothelial cells, cancer cells, motor neurons, and stem cells. ANG is a 14 kDa small protein but possesses three distinct functional sites. As the fifth member of the vertebrate-specific secreted ribonuclease superfamily, ANG has the typical catalytic triad of amino acids (His13, Lye40, and His114) unique to this family of proteins and indeed has ribonucleolytic activity, which is ~105-fold lower than a typical RNase but is essential for most of its biological activities. ANG has a typical nuclear localization sequence (30MRRRG34) that mediates nuclear import after being internalized through receptor-mediated endocytosis. The receptor-binding site of ANG is composed of amino acid residues 59NKNGNPHREN68 and is responsible for interacting with its cell surface receptor plexin-B2.
Figure 1. Properties of angiogenin.
Plexin-B2
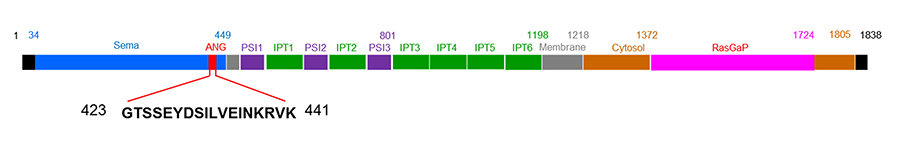
Plexin-B2 (PLXNB2) is the second member of the B class of plexins. Plexins are cell surface receptor for semaphorins (Sema), either alone or in complex with neuropilins. Sema-Plexin interactions are involved in neurogenesis, angiogenesis, tumor progression and immune responses. PLXNB2 was originally identified from malignant brain tumors and is known to play an important role in neuronal development and pattern formation. PLXNB2 expression is widespread during development but is restricted to functionally specialized endothelial cells and certain endocrine organs. We have recently identified PLXNB2 as the functional receptor for ANG that is both necessary and sufficient for the biological functions of ANG including angiogenesis, neurogenesis, neuroprotection, cell growth and survival, as well as regulation of stem cell self-renewal, quiescence, and differentiation. We have pinpointed the ANG-binding site of PLXNB2 (423GTSSEYDSILVEINKRVK441) and have used this peptide as the immunogen to generate a series of monoclonal antibodies. These anti-PLXNB2 mAbs are able to block ANG binding to PLXNB2, abolish nuclear translocation and stress granule accumulation of ANG, decrease ANG-mediated rRNA transcription and tiRNA production, and attenuate the growth and survival functions of ANG. They are being humanized for therapeutic purposes.
Figure 2. Schematic view of ANG binding site and domain structures of PLXNB2.
Mechanism of Action of ANG
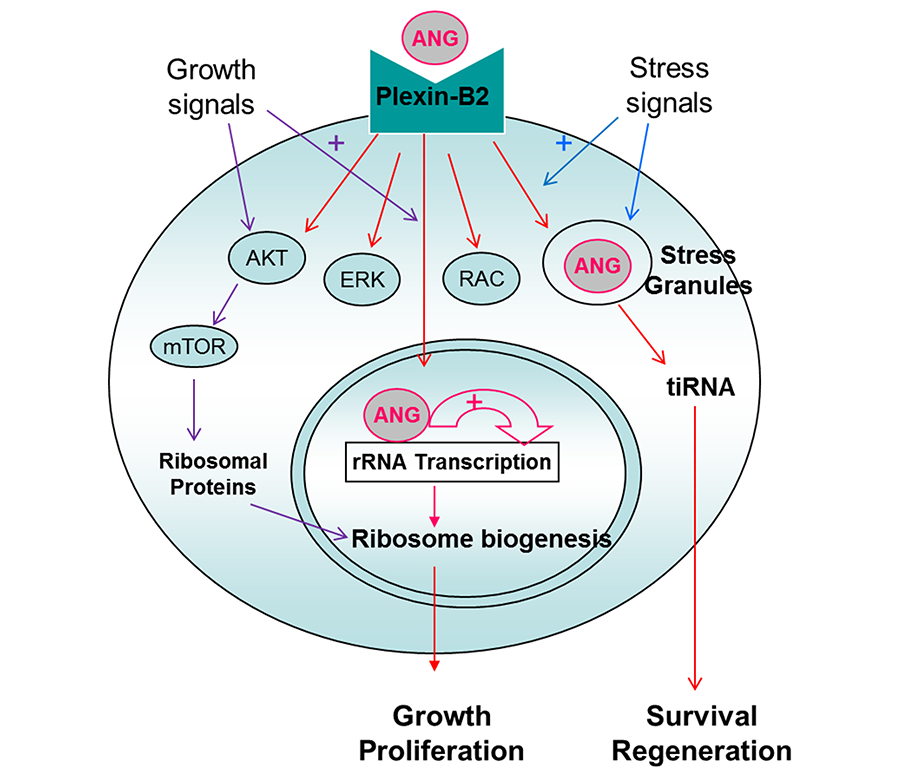
ANG binds to PLXNB2, elicits classical signaling events including AKT and ERK phosphorylation, as well as RAC and CDC42 activation. A unique feature of ANG, which is distinct from other growth factors, is that ANG is internalized and translocated to either nucleus or stress granules, dependent on cell status and environmental conditions. When cells are actively growing and proliferating, ANG is translocated to the nucleus to promote rRNA transcription to meet the high metabolic demand of these cells for protein translation. When cells are stressed, ANG is translocated to stress granules and is associated with tiRNA production to enhance damage repairs and to promote cell survival. tiRNA suppresses global protein translation to save anabolic energy, and at the same time permits IRES-mediated protein translation, a mechanism often used by anti-apoptotic and pro-survival genes.
This dichotomous regulation of ANG toward protein translation is also found in hematopoietic stem and progenitor cells (HSPC). In the most primitive hematopoietic stem cells (HSC) including LT-HSC, ST-HSC, and a subset of MPP, ANG enhances quiescence by enhancing tiRNA production and restricting protein translation. In more differentiated myeloid progenitor (MyePro) cells, ANG stimulates proliferation by promoting rRNA transcription and increasing protein translation. Consistently, ANG is translocated to stress granules in HSC but to nucleus in MyePro. It is unclear at present which determine differential subcellular localization of ANG, but available evidence suggest that ribonuclease/angiogenin inhibitor 1 (RNH1), an abundant intracellular protein that binds ANG in femtomolar affinity, play an important role in this process.
Figure 3. Mechanism of action of ANG
ANG Promotes Hematopoietic Regeneration and Enhances Stem Cell Transplantation
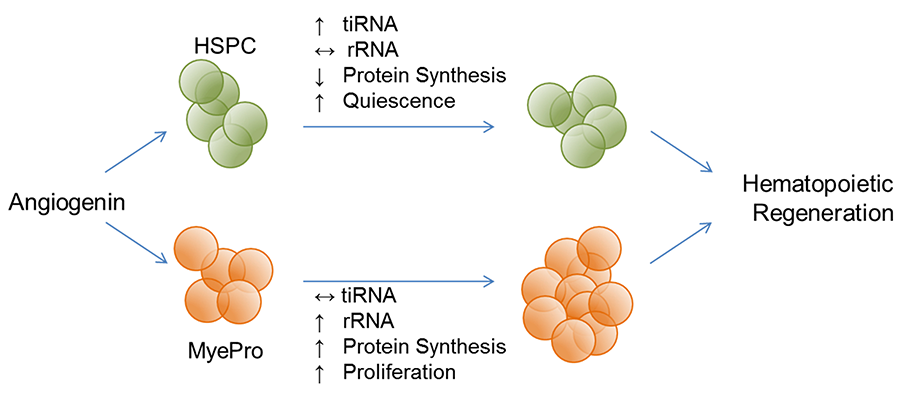
Functional hematopoiesis depends on two cell populations: a rapidly responding lineage-restricted progenitor cell pool and a long-term regenerating hematopoietic stem cell reserve. Functionally, ANG promotes hematopoietic regeneration by preserving the stemness of primitive HSPC while simultaneously promoting expansion of MyePro cells. We have shown that 1) young Ang-/- mice have lymphocytosis but aged Ang-/- mice develop leukopenia; 2) LT-HSC in Ang-/- mice are less quiescent, leading to defective HSC transplantation and complete exhaustion upon secondary transplantation; 3) while HSPC in Ang-/- mice cycle more actively, Ang-/- MyePro cells have more restricted cell cycling; 4) Ang-/- mice have increased susceptibility to treatment with 5-FU and gamma irradiation, accompanied by a decrease in BM cellularity, HSC number, more active cycling, and increased apoptosis.
Mechanistically, ANG regulates hematopoiesis and hematopoietic regeneration through a non-cell autonomous manner. We have shown that 1) conditional knockout of Ang gene in HSC niche cell types, including Osx+ osteoprogenitors, nestin+ mesenchymal progenitors, NG2+ periartetiolar sheath cells phenocopy straight Ang knockout in hematopoiesis, in HSPC and MyePro cycling and in post-transplantation defect; 2) the dichotomous effect of ANG on HSC and MyePro proliferation is mediated by PLXNB2. Conditional knockout of Plxnb2 in Mx1+ hematopoietic cells resembles Ang-/- mice in phenotypic and functional defects of hematopoiesis. Thus, niche-produced ANG and HSPC-located PLXNB2 constitute a non-cell autonomous regulation of HSC stemness.
Therapeutically, we have shown that 1) administration of recombinant ANG protein 24 h prior and post irradiation increased survival of WT and Ang-/- mice, accompanied by a recovery of radiation-induced loss of BM cellularity and an increase of quiescent HSC pool as well as reduced cycling of HSPC and increased cycling of MyePro; 3) ex vivo treatment of both mouse and human HSC with ANG improves transplantation efficiency, in association with restricted cell proliferation. These findings indicate that while ANG treatment improves pro-survival functionality and reduces apoptotic activity in both HSC and MyePro, it restricts proliferation in HSC but enhances it in MyePro. Thus, ANG simultaneously protects against HSC exhaustion, and promotes MyePro expansion for both long-term and short-term reconstitution, a unique feature amendable for bone marrow failure therapy.
Figure 4. ANG dichotomously regulates quiescence and proliferation of stem and progenitor cells
ANG Protects Neuron Degeneration and Prolongs Survival of ALS Model Mice
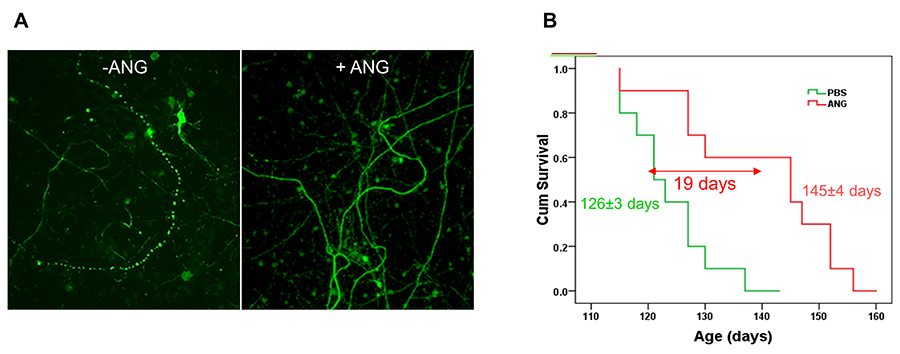
Missense mutations in the coding region of ANG have been identified in ALS patients since 2006. Biochemical studies of the ALS-associated ANG variants indicate that they are defective in one or multiple activities including ribonucleolytic activity, nuclear translocation, and cellular secretion, which result in an impairment of its biological function. Indeed, ANG-associated ANG variants fail to produce rRNA and tiRNA, fail to stimulate angiogenesis and neurogenesis, and fail to protect stress-induced neurofilamant fragmentation. These findings implicated ANG as a loss-of-function mutated gene in ALS, and suggested that ANG protein supplementary therapy might be beneficial in ALS treatment. Indeed, we and others have shown that recombinant human ANG protein has been found to prevent motor neuron degeneration in vitro, and to prolong survival of both SOD1G93A and SOD1G37R mice in vivo.
Figure 5. ANG protects stress-induced neurofilament fragmentation (A) and enhances survival of SOD1G93A mice (B).
ANG-PLXNB2 are Molecular Targets for Cancer Drug Development
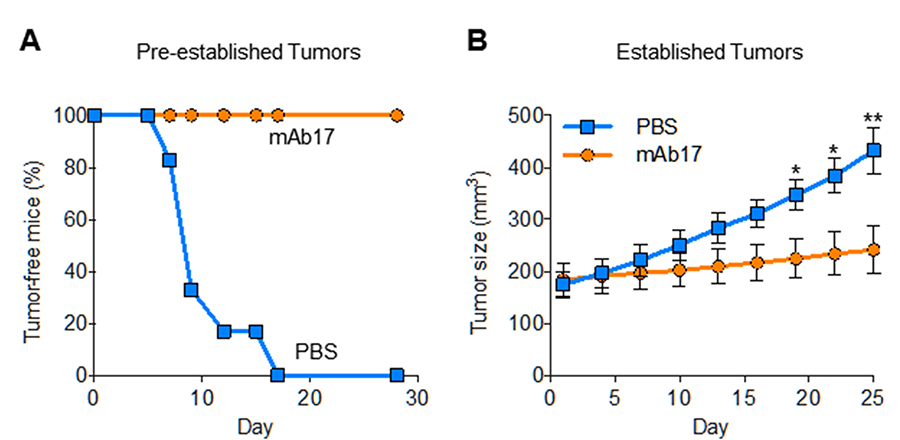
Since ANG was identified as a tumor angiogenic factor and that ANG is universally upregulated in human cancers, there has been extensive effort to develop ANG inhibitors as cancer therapeutics. ANG inhibitors including monoclonal antibodies that neutralize ANG, small molecules that inhibit the ribonucleolytic activity and that blocks nuclear translocation of ANG, as well as antisense DNA and siRNA that inhibit ANG synthesis, have all been shown to inhibit tumor growth in both xenograft and genetic animal models. However, the receptor for ANG had remained unknown despite extensive efforts, and the lack of mechanistic comprehension prevented further development of ANG inhibitors in cancer therapy. Identification and characterization of PLXNB2 as the functional ANG receptor in cancer and endothelial cells not only provides much needed mechanistic understanding for further preclinical development but also offers a novel molecular target for intervention in cancer therapy. In this regard, we have shown that mAb17, which targets the ANG-binding site of PLXNB2, has robust anti-cancer activity in mice without significant toxicities.
Figure 6. mAb17 prevents establishment of PC-3 xenografts (A) and inhibits growth of established PC-3 xenograft tumors (B) in athymic mice.