The Ana Soto Lab
A New Theory of Carcinogenesis
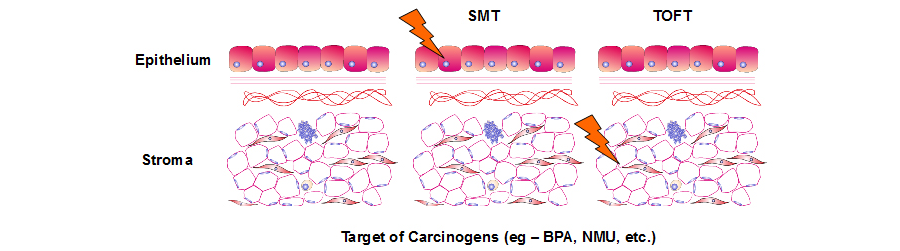
The predominant theory in the field of cancer research, the somatic mutation theory (SMT) considers cancer as cell-based disease. The basic premises of SMT are 1) cancer is a defect of the control of cell proliferation and 2) the default state of metazoan cells is quiescence. Instead, we propose an alternative theory, the tissue organization field theory (TOFT) whose basic premises are 1) proliferation and motility are the default states of all cells, and 2) cancers/neoplasia are defects of tissue architecture, and thus cancer is a tissue-based disease. Carcinogens act initially by disrupting the normal interactions that take place among cells in the parenchyma and stroma of an organ (the equivalent of the “morphogenetic fields” of developing organisms). This disruption results in a lessening of the cells' ability to “read” their positional and historical background and a change in the appearance of the tissue. For example, the tissue might become hyperplastic, dysplastic or metaplastic. Finally, the epithelium develops carcinoma in situ.
Figure 1. In the premises of TOFT, carcinogens target the epithelia and the stroma to induce tumor formation.
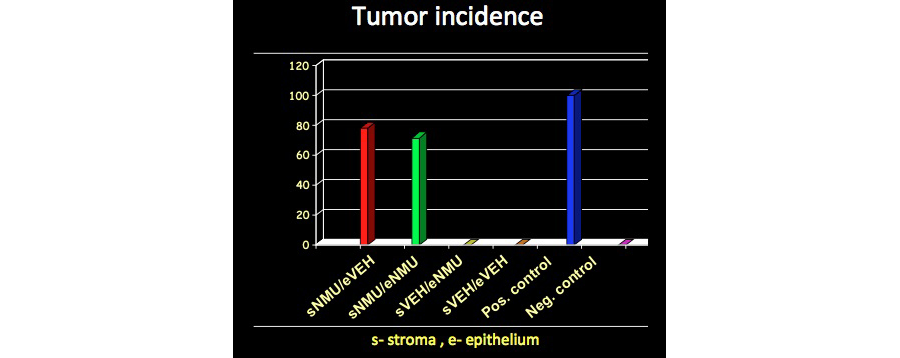
This novel theory incorporates the notion that neoplastic cells may behave like “normal” cells when placed within normal tissues. Using a theory-neutral design, we tested three competing hypotheses, namely, 1) the primary target of carcinogens is the stroma, 2) the primary target is the epithelium and 3) both stroma and epithelium need to be exposed to the carcinogen. We observed that the recombination of a stroma exposed to a carcinogen (N-nitroso-N-methylurea, NMU) with normal epithelial cells resulted in neoplasms, but the reverse combination did not. This implies that the stroma, rather than individual cells of the epithelium, is the target of the carcinogen. These results also suggested that the neoplastic phenotype is contextual and hence, potentially reversible. We explored this hypothesis as well and found that mammary neoplastic cells isolated from tumors could be normalized when recombined with the stroma or normal mature hosts.
Figure 2 – Tumors only develop in NMU-treated stroma of mammary glands. (Maffini et al 2004)
Self-organization to Morphogenesis
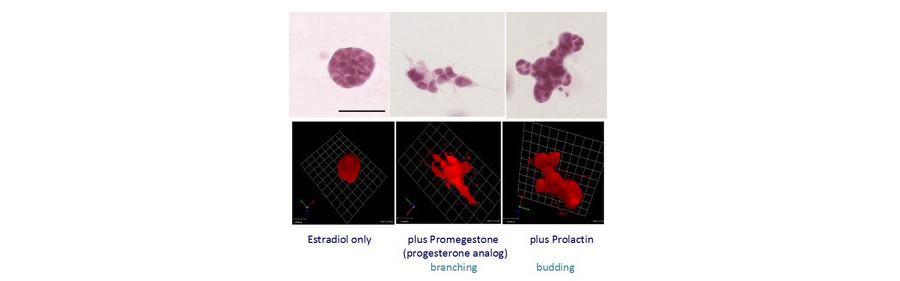
Our laboratory is interested in observing cancer in statu nascendi and to that end we are using 3D culture models to study cell-cell and cell-matrix interactions during normal tissue development and in carcinogenesis. Since we hypothesize that cancer is a case of development gone awry, we are studying the development and morphogenesis of the mammary gland, our experimental model using these models. We have developed a novel, hormone-sensitive 3D culture model to study the effects of specific reproductive hormones (estrogen, progesterone and prolactin) on mammary epithelial morphogenesis. In our model, we have shown that estrogen addition results in ductal elongation, estrogen + progesterone results in its branching and estrogen + prolactin results in budding of the epithelial cells embedded in a 3D collagen gel (Fig 3).
Figure 3. T47D breast cancer cells embedded in collagen gels stained with carmine alum. (Top) Representative structures formed by T47D cells by hormone treatment (Bottom). Reconstructed 3D projections of epithelial structures from z-stack images using Volocity software. (Speroni et al 2014)
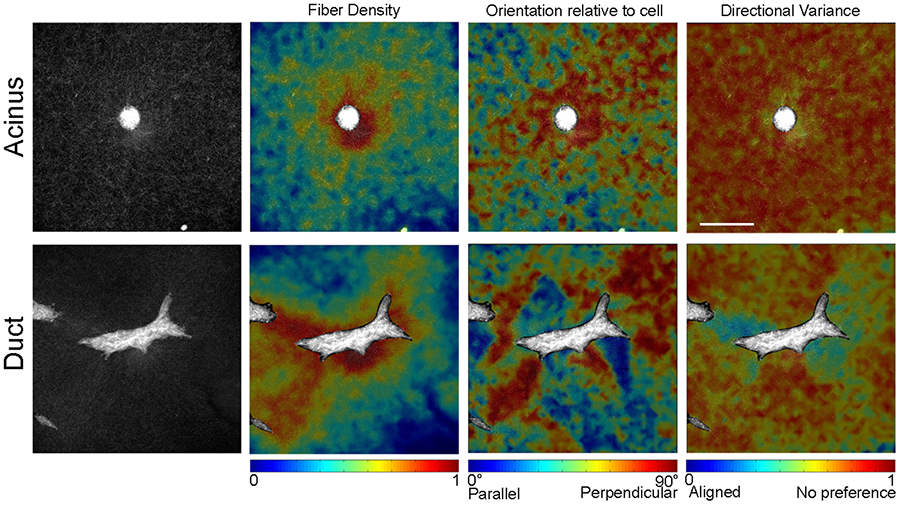
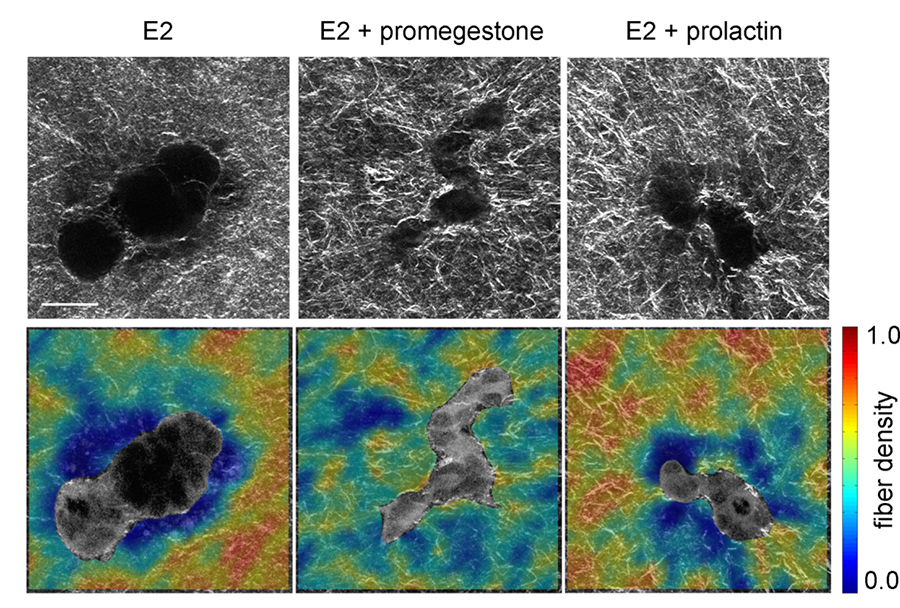
The development of the mammary gland is largely dictated by stromal-epithelial interactions. Biomechanical factors, such as matrix stiffness, have been shown to be key players in such interactions. Type I collagen is the predominant collagen type in the mammary gland and collagen fiber organization has been shown to be important for normal mammary gland development. In our 3D in vitro culture model, we have shown that collagen fiber organization changes before epithelial cells start to organize into structures such as ducts or acini. Hormonal treatment also resulted in altered collagen fiber organization around epithelial structures.
Figure 4. Collagen fiber organization around MCF10A acinus and duct in 3D gels. Fiber alignment was visualized using reflectance confocal microscopy. (Barnes et al 2014)
Figure 5. Collagen fiber organization around epithelial structures formed by T47D cells by hormonal treatment. Fiber organization visualized using second harmonic generation imaging. (Speroni et al 2014)
Endocrine Disruption
A related topic of investigation in our laboratory is endocrine disruption, which we came upon accidentally in the late 1980s. Since then, we have identified several novel environmental estrogens (xenoestrogens) such as nonyl-phenol (a widely used antioxidant and detergent), plasticizers such as dibutylphthalate and benzylbutylphthalate and pesticides such as dieldrin, toxaphene and endosulfan. We have also developed a bio-assay for screening xenoestrogens, the E-SCREEN assay that is now used worldwide. Our findings indicate that these xenoestrogens may have a causal role in increasing the incidence of human diseases and conditions like reproductive impairment, cancer, obesity, diabetes and altered behaviors.
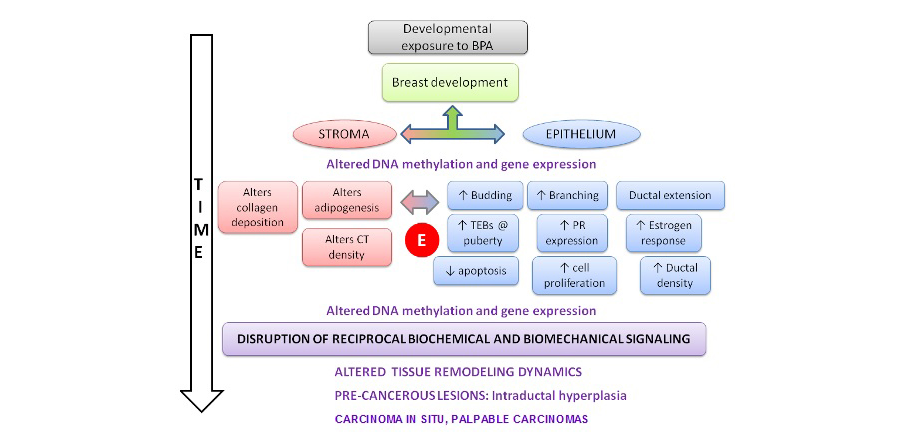
Figure 6. Timeline of BPA's effects and processes affected in mammary gland development (Maffini et al 2011)
Our current work in this field focuses on bisphenol-A (BPA) and how it might lead to the conditions as listed above. We have shown that in utero exposure to minute and environmentally relevant levels of BPA irreversibly alters the development of the female genital tract and the mammary gland, induces obesity and alters sexually dimorphic behavior and brain structures. We are currently exploring the processes that may explain these effects. In the mammary gland we are examining the reciprocal interactions between stroma and epithelium which include biochemical mediators resulting from altered DNA methylation and gene expression and biophysical mediators such as altered collagen fiber organization, altered biophysical properties of the extracellular matrix, at different stages of development from the fetal to the adult. Additionally, we are exploring the processes that lead to the obesogenic effect of BPA. We have found that the metabolomic profile is altered already during the neonatal period and that metabolic effects persist at all ages examined. This work is being pursued in collaboration with with Drs. Beverly Rubin (Tufts University School of Medicine), Daniel Zalko, and Fabien Jourdan(National Institute of Agricultural Research, Paris, France).
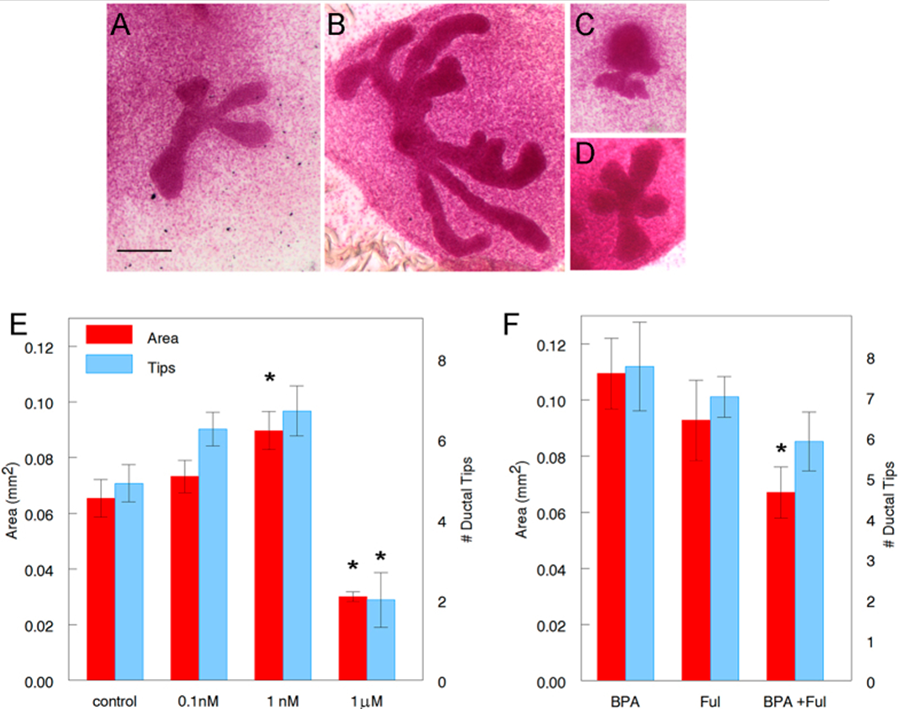
Figure 7. Effect of BPA on fetal mammary glands cultured ex vivo. Carmine stained whole mounts of cultured mammary explants in (A) Control (CDFBS), (B) 1 nM BPA, (C) 1 μM BPA and (D) 1 nM BPA + 100 nM Ful. Scale bar: 200 μm. (E) Ductal area and number of ductal tips of mammary buds treated with BPA compared to control. Asterisk denotes significance compared with control. Data from three independent experiments, n = 22 for area and n = 21 for number of ductal tips in control, n = 12 in 0.1 nM, n = 20 in 1 nM and n = 8 for area and n = 9 for number of ductal tips in 1 μM group. (F) Effect of Ful (100 nM) on BPA (1 nM) -treated mammary bud cultures. Asterisk denotes a statistically significant difference from BPA alone. Data from three independent experiments, n = 9 for area and n = 8 for number of ductal tips in BPA and in Ful and n = 10 in BPA + Ful.
Theoretical Biology and Philosophy of Biology
Drs. Soto and Sonnenschein are deeply invested in understanding the theoretical bases of experimental biology, especially epistemological issues in Cancer Biology. To that end, Dr. Soto, during her tenure at Ecole Normale Superieure in Paris as the prestigious Blaise Pascale Chair, worked on a theory of organisms that covers the entire lifecycle. This work was done by the ORGANISM group including Drs. Soto and Sonnenschein and a group of mathematicians and philosophers (G. Longo, M. Montevil, M. Mossio, N. Perret, A. Pocheville, PA Miquel). They proposed three founding principles: 1) the default state of unicellular organisms as well as of cells from multicellular organisms is proliferation with variation and motility, 2) the principle of organization by closure of constraints, and 3) a principle of variation. The first deals with the ability of living organisms to generate action (agency) and to create their own rules (normativity). The second principle explains robustness and stability, and the third principle explains plasticity novelty. Papers outlining the theoretical, philosophical, mathematical & experimental underpinnings of such a position have been published in a special issue of Progress in Biophysics & Molecular Biology. They are currently working together with the other member of the ORGANISM group on more fully developing this theory, while also defining new biological observables and an appropriate language to describe them.