The Athar Chishti Lab
Pathophysiology of Blood Diseases and Global Health
We study cytoskeletal and signaling proteins in blood disorders, including malaria, babesiosis, sickle cell disease, and thrombosis.
Host Parasite Interactions
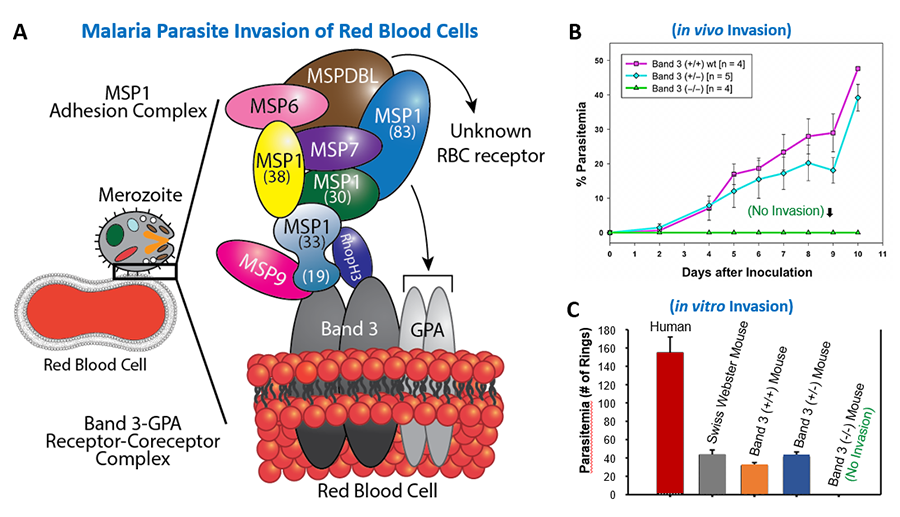
We investigate the mechanisms of parasite infection in human red blood cells (erythrocytes) with the expectation of unveiling novel therapeutic targets in cerebral and placental malaria. Molecular understanding of parasite proteins and their cognate host receptors is essential for the development of new therapeutics as well as an effective multi-subunit vaccine. We have identified an essential role of an erythrocyte membrane complex consisting of Band 3 (anion exchanger-1 or solute carrier family 4 member1; SLC4A1) and Glycophorin A (sialoglycoprotein; GPA) during the entry of malaria parasite in erythrocytes. This was accomplished using a multidisciplinary approach, including the phage display cDNA technology and unique mouse models. Currently, we are investigating parasite-derived secretory proteins that mediate the adhesion of infected erythrocytes to human endothelial cells. Using innovative phage display cDNA screening approaches, we have recently identified multiple immunoreactive antigens in Babesia microti, a malaria-like protozoan, that is transmitted by deer ticks. We plan to combine immunodominant antigens for the development of diagnostic assays with the ultimate goal of generating a vaccine against Babesiosis.
Figure1. Host-Parasite Interactions. (A) Red blood cell (RBC) invasion by the human malaria parasite Plasmodium falciparum. (B) Resistance of host Band 3 and GPA deficient mice to malaria infection in vivo. (C) P. falciparum invasion of human and mouse RBCs in vitro. No invasion in Band 3 and GPA deficient mouse RBCs. For details, refer to Baldwin MR, et al, 2015. Blood 125: 2704-2711. (Abstract).
Calcium Signaling in Sickle Cell Disease and Thrombosis
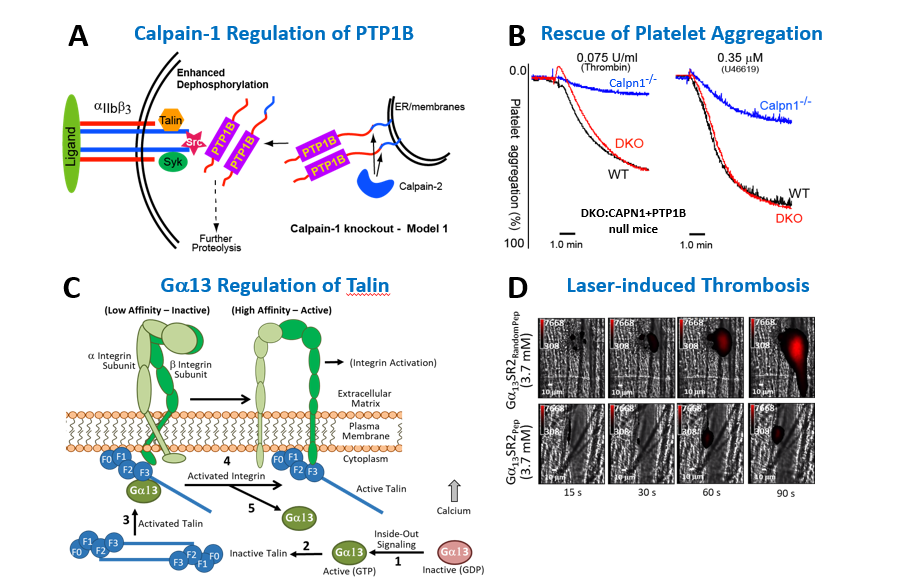
We have a long track record in the biology of calpain-1, a calcium-dependent cysteine protease, which regulates multiple signaling and cytoskeletal cascades. Our laboratory generated the first mouse model lacking calpain-1 activity (CAPN1 null) and identified its physiological substrates, including Protein Tyrosine Phosphatase 1B (PTP1B) (or PTPN1). Using CAPN1 and PTP1B double knockout mice, we demonstrated a functional role of calpain-1 in platelet secretion, adhesion, and shape change pathways. Currently, we are investigating the function of calpain-1 in sickle cell disease (SCD). A hallmark of SCD is elevated calcium and thrombotic lesions. Using a CAPN1 null humanized mouse model of SCD (Townes Model) developed in our laboratory, we are clarifying the role of calpain-1 and calcium-mediated protein degradation in erythrocyte shape change, chronic pain, and thrombosis directly relevant to patients with SCD.
Figure 2. Calcium Signaling in Sickle Cell Disease and Thrombosis. (A) Model for the regulation of PTP1B by calpains. (B) Correction of the platelet aggregation defect in double knockout mice lacking calpain-1 and PTP1B. (C) Gα13-Talin-Integrin activation pathway in Platelets. Gα13 directly binds to the F3 domain of talin to relieve its autoinhibited state. (D) Laser-induced thrombosis of mice pre-treated with myristoylated G13-SR2 scrambled peptide (top panels) or G13-SR2 active peptide (bottom panels). For details, refer to Kuchay et al, 2007. Mol. Cell Biol. 27: 6038-6052. (Abstract) and Schiemer J, et al, 2016. J Biol Chem. 291: 26598-26612. (Abstract).
Scaffolding Proteins and Protein Transport
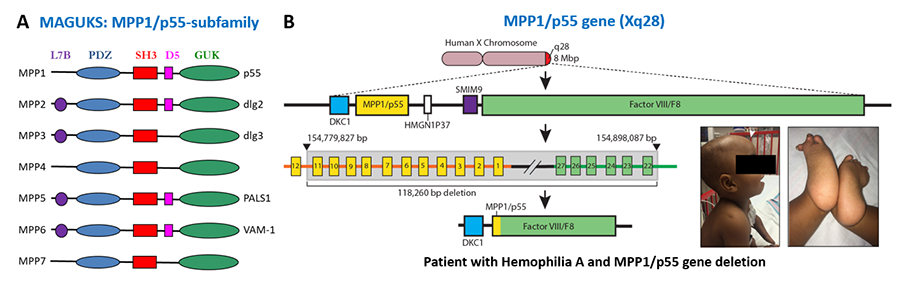
Membrane-associated guanylate kinase homologues (MAGUKs) are scaffolding proteins composed of PDZ, SH3, and GUK domains that are essential for the maintenance of cell polarity. MAGUK family traces its origin to the cloning of Drosophila discs-large tumor suppressor gene and human erythrocyte p55/MPP1 gene. Our subsequent studies identified the MAGUK-Cytoskeletal interface first elucidated by the direct binding of MPP1 to the FERM domain. Moreover, we identified a new mechanism of intracellular transport of PIP3, a product of phosphatidylinositol 3-kinase, mediated by the hDlg and kinesin-3 family motor protein GAKIN, now called KIF13B. Recently, we reported the first human patient with complete MPP1/p55 deficiency afflicted with severe developmental defects. Currently, we are investigating the function of p55/MPP1 in vesicle transport and lipid homeostasis.
Figure 3. Scaffolding Proteins and Protein Transport. (A) Domain organization of membrane associated guanylate kinase homologues (MAGUKs). (B) A patient with Hemophilia A and severe developmental defects with Xq28 chromosomal deletion including Factor VIII/F8 and MPP1/p55. For details, refer to Fritz et al, 2019. Am J Hematol. 94: E29-E32. (Abstract)