The Xudong Li Lab
Control of CD4 T Cell Identity by Cis-regulatory DNA Elements
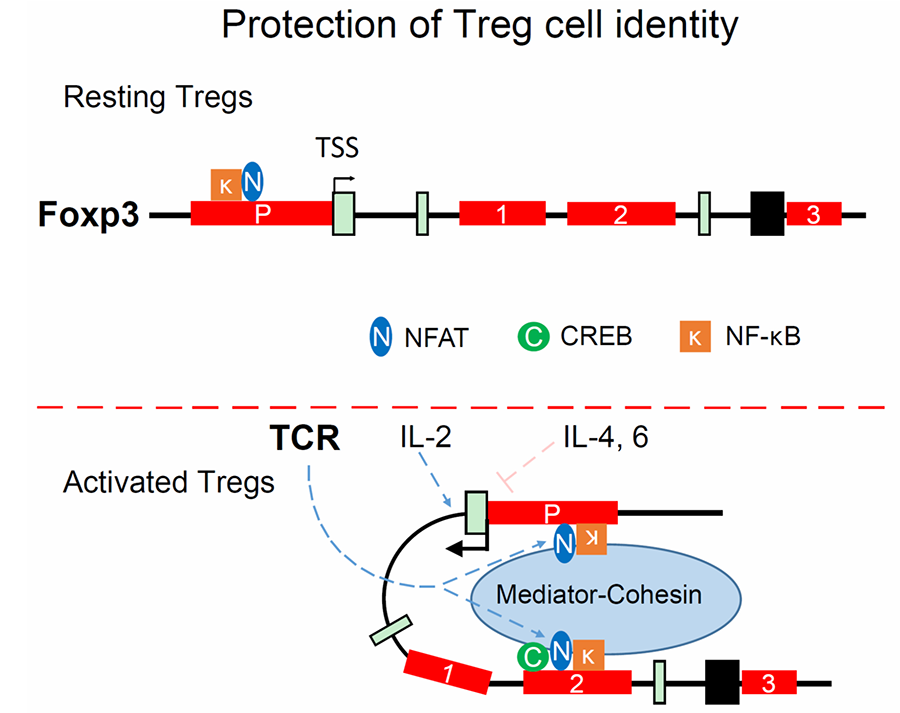
Immune homeostasis depends on coordinated activities of many types of immune cells, including various subtypes of CD4 helper T cells in the adaptive immune system. Their cellular identities are controlled by immune signals and other environmental stimuli. However, understanding how diverse signals are integrated to shape not only the differentiation, but also the lineage stability and plasticity of CD4 T cells remains a big challenge. We posited that cis-regulatory DNA elements can control distinct aspects of CD4 T cell identities by acting as discrete sensor hubs that integrate diverse cellular signals to regulate the expression of cellular identity-defining genes. To this end, we studied the role of CNS2, a CpG-rich conserved enhancer of the Foxp3 gene, in controlling the identity of regulatory T cells (Tregs). Using genetically modified mice deficient of CNS2, we discovered that CNS2 plays an indispensable role in protecting the identity of Tregs and elucidated the molecular mechanism of CNS2-mediated protection (Figure 1). Therefore, an enhancer (CNS2) can use its epigenetic status (CpG methylation) to contextualize and integrate diverse cellular signals (TCR and cytokine) to control a distinct aspect (lineage stability) of Treg cellular identity. We are interested in examining the roles of other cis-regulatory DNA elements in controlling CD4 T cell identities by employing cutting-edge genetic, biochemical, and immunological approaches. The results of these studies will shed light on how dynamic regulation of CD4 T cell identities promotes immune homeostasis and how “identity crisis” leads to immunological disorders.
Figure 1. A model of CNS2-mediated protection of the cellular identity of regulatory T cells (Tregs). In resting Tregs, CNS2 is not required for stabilizing Foxp3 expression. In Tregs that are undergoing functional adaptation, CNS2 interacts with the Foxp3 promoter in a NFAT-dependent manner to prevent the destabilization of Foxp3 expression by pro-inflammatory cytokines such as IL-4 and IL-6.
Molecular Mechanisms of CD4 Helper T cell Functional Adaptation
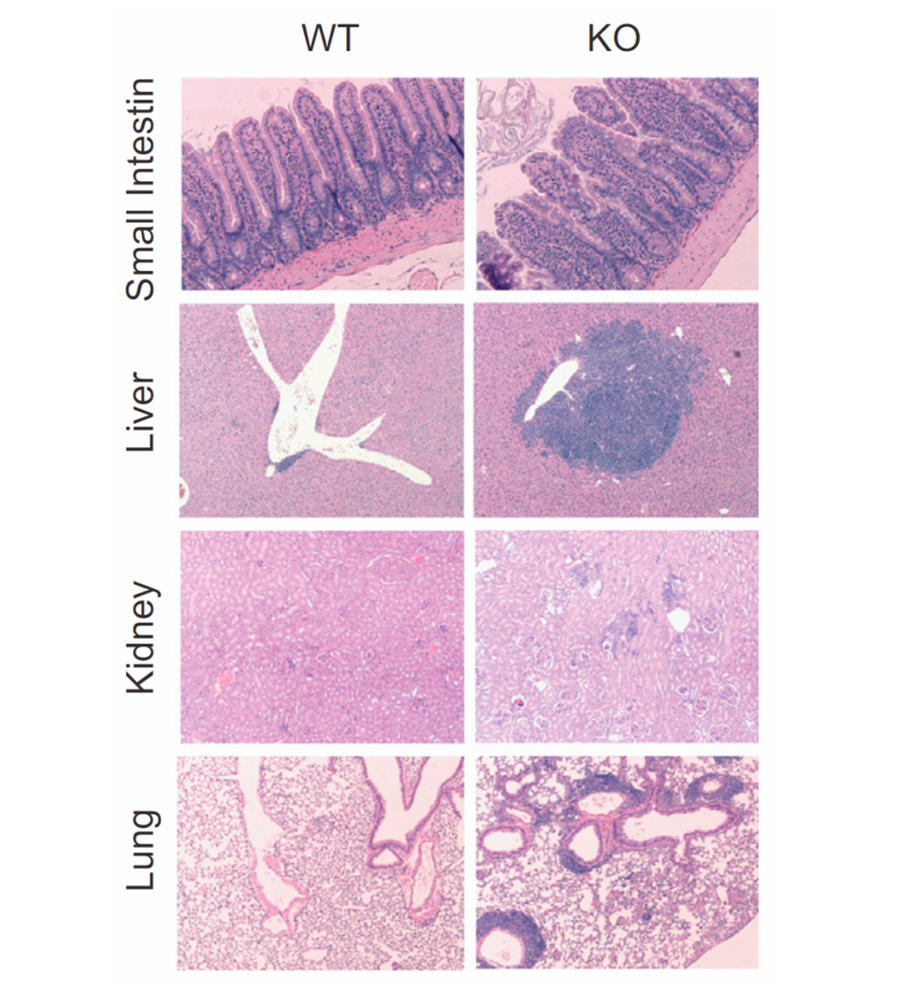
Emerging evidence supports the existence of various functional states within an immune cell type. However, the nature of these functional states, the molecular mechanisms governing their formation and transition, and their contributions to immune homeostasis are not well understood. We postulated that functional states of CD4 T cells are dynamically regulated and yet metastable functional programs established in differentiated CD4 T cells in response to environmental stimuli, and that they allow for spatiotemporal adaptation of CD4 T cell functions to diverse tissue and immune microenvironments. Furthermore, we hypothesized that stable cellular identities can provide a foundation for functional adaptation, but may also constrain the spectrum of functional states within a given cell type. Conversely, functional adaptation may challenge the stability of cellular identities that in turn require active protective mechanisms. Indeed, we found that CNS2 deletion led to destabilization of Tregs undergoing functional adaptation and resulted in tissue inflammation (Figure 2). Therefore, our study not only established an essential role for the functional adaptation of Tregs in suppressing inflammation under diverse tissue and inflammatory conditions, but also revealed a critical link between the cellular identity and functional states of Tregs. We are interested in exploring molecular mechanisms governing the functional adaptation of CD4 T cells to microenvironments in diverse tissue and immune settings. A better mechanistic understanding will facilitate the development of novel means of therapeutic manipulation of these cells.
Figure 2. Increased inflammation in the small intestine, liver, kidney, and lung of CNS2-deficient mice as a result of impaired ability of Tregs to undergo functional adaptation without losing their cellular identity.